Abstract
Background
Morphine is commonly used for postoperative analgesia in children. Here we studied the pharmacodynamics of morphine in children after cardiac surgery receiving protocolized morphine.
Methods
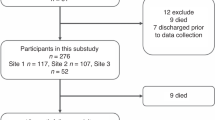
Data on morphine rescue requirements guided by validated pain scores in children (n = 35, 3–36 months) after cardiac surgery receiving morphine as loading dose (100 μg kg−1) with continuous infusion (40 μg kg−1 h−1) from a previous study on morphine pharmacokinetics were analysed using repeated time-to-event (RTTE) modelling.
Results
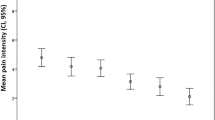
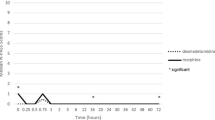
During the postoperative period (38 h (IQR 23–46)), 130 morphine rescue events (4 (IQR 1–5) per patient) mainly occurred in the first 24 h (107/130) at a median morphine concentration of 29.5 ng ml−1 (range 7–180 ng ml−1). In the RTTE model, the hazard of rescue morphine decreased over time (half-life 18 h; P < 0.001), while the hazard for rescue morphine (21.9% at 29.5 ng ml−1) increased at higher morphine concentrations (P < 0.001).
Conclusions
In this study on protocolized morphine analgesia in children, rescue morphine was required at a wide range of morphine concentrations and further increase of the morphine concentration did not lead to a decrease in hazard. Future studies should focus on a multimodal approach using other opioids or other analgesics to treat breakthrough pain in children.
Impact
-
In children receiving continuous morphine infusion, administration of rescue morphine is an indicator for insufficient effect or an event.
-
Morphine rescue events were identified at a wide range of morphine concentrations upon a standardized pain protocol consisting of continuous morphine infusion and morphine as rescue boluses.
-
The expected number of rescue morphine events was found to increase at higher morphine concentrations. Instead of exploring more aggressive morphine dosing, future research should focus on a multimodal approach to treat breakthrough pain in children.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weisman, S. J., Bernstein, B. & Schechter, N. L. Consequences of inadequate analgesia during painful procedures in children. Arch. Pediatr. Adolesc. Med. 152, 147–149 (1998).
Zeilmaker-Roest, G. A. et al. An international survey of management of pain and sedation after paediatric cardiac surgery. BMJ Paediatr. Open 1, e000046 (2017).
Krekels, E. H., Tibboel, D., Danhof, M. & Knibbe, C. A. Prediction of morphine clearance in the paediatric population: how accurate are the available pharmacokinetic models? Clin. Pharmacokinet. 51, 695–709 (2012).
Elkomy, M. H. et al. Pharmacokinetics of morphine and its metabolites in infants and young children after congenital heart surgery. AAPS J. 18, 124–133 (2016).
Valkenburg, A. J. et al. Pharmacodynamics and pharmacokinetics of morphine after cardiac surgery in children with and without Down syndrome. Pediatr. Crit. Care Med. 17, 930–938 (2016).
Coffman, B. L., Rios, G. R., King, C. D. & Tephly, T. R. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 25, 1–4 (1997).
Anand, K. J. et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br. J. Anaesth. 101, 680–689 (2008).
Valitalo, P. A. et al. Morphine pharmacodynamics in mechanically ventilated preterm neonates undergoing endotracheal suctioning. CPT Pharmacometrics Syst. Pharmacol. 6, 239–248 (2017).
Elkomy, M. H. et al. Pharmacodynamic analysis of morphine time-to-remedication events in infants and young children after congenital heart surgery. Clin. Pharmacokinet. 55, 1217–1226 (2016).
Juul, R. V. et al. Repeated time-to-event analysis of consecutive analgesic events in postoperative pain. Anesthesiology 123, 1411–1419 (2015).
Junker, U. & Wirz, S. Influence of circadian rhythms on the therapy of severe pain. J. Oncol. Pharm. Pract. 16, 81–87 (2010).
Hutmacher, M. M. & Kowalski, K. G. Covariate selection in pharmacometric analyses: a review of methods. Br. J. Clin. Pharmacol. 79, 132–147 (2015).
Goulooze, S. C., Valitalo, P. A. J., Knibbe, C. A. J. & Krekels, E. H. J. Kernel-based visual hazard comparison (kbVHC): a simulation-free diagnostic for parametric repeated time-to-event models. AAPS J. 20, 5–9 (2017).
Martini, C. et al. Pharmacokinetic-pharmacodynamic modeling in acute and chronic pain: an overview of the recent literature. Expert Rev. Clin. Pharmacol. 4, 719–728 (2011).
Anderson, B. J. & Holford, N. H. in Pain in Neonates and Infants: Pain Research and Clinical Management Series (eds Anand, K. J., Stevens, P. B. & McGrath, P.) 128 (Elsevier, 2009).
Lynn, A. M., Nespeca, M. K., Opheim, K. E. & Slattery, J. T. Respiratory effects of intravenous morphine infusions in neonates, infants, and children after cardiac surgery. Anesth. Analg. 77, 695–701 (1993).
Bouwmeester, N. J. et al. Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br. J. Anaesth. 90, 642–652 (2003).
Roberts-Thomson, I. C., Jonsson, J. R., Pannall, P. R. & Frewin, D. B. Morphine responders with unexplained pain after cholecystectomy may have sympathetic overactivity. Clin. Auton. Res 1, 59–62 (1991).
Riley, J. et al. No pain relief from morphine? Individual variation in sensitivity to morphine and the need to switch to an alternative opioid in cancer patients. Support Care Cancer 14, 56–64 (2006).
Gram, M. et al. Prediction of postoperative opioid analgesia using clinical-experimental parameters and electroencephalography. Eur. J. Pain 21, 264–277 (2017).
Aubrun, F., Mazoit, J.-X. & Riou, B. Postoperative intravenous morphine titration. Br. J. Anaesth. 108, 193–201 (2012).
Mazoit, J. X., Butscher, K. & Samii, K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth. Analg. 105, 70–78 (2007).
Lötsch, J. Pharmacokinetic-pharmacodynamic modeling of opioids. J. Pain. Symptom Manag. 29, S90–S103 (2005).
Duedahl, T. H. & Hansen, E. H. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatr. Anaesth. 17, 756–774 (2007).
Saini, A., Maher, K. O. & Deshpande, S. R. Nonopioid analgesics for perioperative and cardiac surgery pain in children: current evidence and knowledge gaps. Ann. Pediatr. Cardiol. 13, 46–55 (2020).
Zeilmaker-Roest, G. A. et al. Intravenous morphine versus intravenous paracetamol after cardiac surgery in neonates and infants: a study protocol for a randomized controlled trial. Trials 19, 318 (2018).
van Dijk, M. et al. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain 84, 367–377 (2000).
Acknowledgements
The authors thank Cormac V Breatnach, Department of Anaesthesia and Critical Care Medicine, Our Lady’s Children’s Hospital, Dublin, Ireland for his contribution to the original study and Ko Hagoort, Department of Pediatric Surgery, Erasmus MC-Sophia Children’s Hospital, Rotterdam, The Netherlands for text editing. No financial support has been received for this study.
Author information
Authors and Affiliations
Contributions
S.d.H., S.C.G., A.J.V.: substantial contributions to overall concept design, data acquisition, analysis and interpretation, article revision, and final approval. E.H.J.K., M.v.D., D.T., C.A.J.K.: substantial contributions to overall concept design, data interpretation, article revision, and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent for the study was obtained from the parents preoperatively.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
de Hoogd, S., Goulooze, S.C., Valkenburg, A.J. et al. Postoperative breakthrough pain in paediatric cardiac surgery not reduced by increased morphine concentrations. Pediatr Res 90, 1201–1206 (2021). https://doi.org/10.1038/s41390-021-01383-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01383-z