Abstract
Background
Evidence suggests that fronto-limbic brain regions and connecting white matter fibre tracts in the left hemisphere are more sensitive to glucocorticoids than in the right hemisphere. It is unknown whether treatment with glucocorticoids in childhood is associated with microstructural differences of the uncinate fasciculus and cingulum bundle, which connect fronto-limbic brain regions. Here, we tested the hypothesis that prior glucocorticoid treatment would be associated with differences in fractional anisotropy (FA) of the left relative to right uncinate fasciculus and cingulum bundle.
Methods
We performed diffusion-weighted imaging in 28 children and adolescents aged 7–16 years previously treated with glucocorticoids for nephrotic syndrome or rheumatic disease and 28 healthy controls.
Results
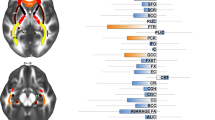
Patients displayed significantly different asymmetry in the microstructure of uncinate fasciculus with higher left but similar right uncinate fasciculus FA and axial diffusivity compared to controls. No apparent differences were observed for the cingulum. Notably, higher cumulative glucocorticoid doses were significantly associated with higher uncinate fasciculus FA and axial diffusivity bilaterally.
Conclusions
Our findings indicate that previous glucocorticoid treatment for non-cerebral diseases in children and adolescents is associated with long-term changes in the microstructure of the uncinate fasciculi, and that higher cumulative glucocorticoid doses have a proportional impact on the microstructure.
Impact
-
It is unknown if treatment with glucocorticoids in childhood have long-term effects on fronto-limbic white matter microstructure.
-
The study examined if children and adolescents previously treated with glucocorticoids for nephrotic syndrome or rheumatic disorder differed in fronto-limbic white matter microstructure compared to healthy controls.
-
The nephrotic and rheumatic patients had higher left but similar right uncinate fasciculus FA and axial diffusivity.
-
Higher bilateral uncinate fasciculus FA and axial diffusivity was associated with higher cumulative glucocorticoid doses.
-
We revealed new evidence suggesting that previous glucocorticoid treatment for non-cerebral diseases in children and adolescents is associated with long-term changes in uncinate fasciculi microstructure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
09 February 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41390-021-01736-8
References
Coutinho, A. E. & Chapman, K. E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 335, 2–13 (2011).
Damsted, S. K., Born, A. P., Paulson, O. B. & Uldall, P. Exogenous glucocorticoids and adverse cerebral effects in children. Eur. J. Paediatr. Neurol. 15, 465–477 (2011).
de Kloet, E. R., Joels, M. & Holsboer, F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 (2005).
Gray, J. D., Kogan, J. F., Marrocco, J. & McEwen, B. S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 13, 661–673 (2017).
de Kloet, E. R. et al. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 57, 1329–1336 (2000).
Harris, A. & Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Hormones Behav. 59, 279–289 (2011).
Jernigan, T. L., Baare, W. F., Stiles, J. & Madsen, K. S. Postnatal brain development: structural imaging of dynamic neurodevelopmental processes. Prog. Brain Res. 189, 77–92 (2011).
Graham, A. M. et al. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry 85, 172–181 (2019).
Scheinost, D., Spann, M. N., McDonough, L., Peterson, B. S. & Monk, C. Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology 45, 1272–1279 (2020).
Burghy, C. A. et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 15, 1736–1741 (2012).
Gee, D. G. et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl Acad. Sci. USA 110, 15638–15643 (2013).
Carrion, V. G., Weems, C. F., Richert, K., Hoffman, B. C. & Reiss, A. L. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biol. Psychiatry 68, 491–493 (2010).
Carrion, V. G., Weems, C. F. & Reiss, A. L. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119, 509–516 (2007).
Davis, E. P., Sandman, C. A., Buss, C., Wing, D. A. & Head, K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry 74, 647–655 (2013).
Ocklenburg, S., Korte, S. M., Peterburs, J., Wolf, O. T. & Gunturkun, O. Stress and laterality - the comparative perspective. Physiol. Behav. 164, 321–329 (2016).
Cerqueira, J. J., Almeida, O. F. & Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 22, 630–638 (2008).
Cerqueira, J. J. et al. Corticosteroid status influences the volume of the rat cingulate cortex - a magnetic resonance imaging study. J. Psychiatr. Res. 39, 451–460 (2005).
Czeh, B. et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 32, 1490–1503 (2007).
Madsen, K. S. et al. Hypothalamic-pituitary-adrenal axis tonus is associated with hippocampal microstructural asymmetry. Neuroimage 63, 95–103 (2012).
Madsen, K. S. et al. Cortisol awakening response and negative emotionality linked to asymmetry in major limbic fibre bundle architecture. Psychiatry Res. 201, 63–72 (2012).
Beaulieu, C. in Diffusion MRI: From Quantitative Measurement to In-Vivo Neuroantomy (eds Johansen-Berg, H. & Behrens, T. E. J.) 105–126 (Elsevier, 2009).
Schmahmann, J. D. et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130, 630–653 (2007).
Lebel, C. & Beaulieu, C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 31, 10937–10947 (2011).
Eddy, A. A. & Symons, J. M. Nephrotic syndrome in childhood. Lancet 362, 629–639 (2003).
Ravelli, A. & Martini, A. Juvenile idiopathic arthritis. Lancet 369, 767–778 (2007).
Holm, S. K. et al. Children and adolescents previously treated with glucocorticoids display lower verbal intellectual abilities. Acta Paediatr. 104, 784–791 (2015).
Holm, S. K. et al. Total brain, cortical, and white matter volumes in children previously treated with glucocorticoids. Pediatr. Res. 83, 804–812 (2018).
Holm, S. K. et al. Previous glucocorticoid treatment in childhood and adolescence is associated with long-term differences in subcortical grey matter volume and microstructure. Neuroimage Clin. 23, 101825 (2019).
Vestergaard, M. et al. Glucocorticoid treatment earlier in childhood and adolescence show dose-response associations with diurnal cortisol levels. Dev. Psychobiol. 59, 1010–1020 (2017).
Reese, T. G., Heid, O., Weisskoff, R. M. & Wedeen, V. J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 49, 177–182 (2003).
Jovicich, J. et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30, 436–443 (2006).
Andersson, J. L. R., Hutton, C., Ashburner, J., Turner, R. & Friston, K. Modeling geometric deformations in EPI time series. Neuroimage 13, 903–919 (2001).
Smith, S. M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Jones, D. K. & Basser, P. J. “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magn. Reson. Med. 52, 979–993 (2004).
Cook, P. A. et al. Camino: open-source diffusion-MRI reconstruction and processing. In 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA, p. 2759 (2006).
Smith, S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 (2006).
Andersson, L. L. R., Jenkinson, M. & Smith, S. Non-linear Registration, aka Spatial Normalisation. FMRIB technical report no. TR07JA2 (FMRIB Centre, 2007).
Mori, S., Wakana, S., Nagae-Poetscher L. M. & Van Zijl, P.C.M. MRI Atlas of Human White Matter 1st edn (Elsevier, 2005).
Hua, K. et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347 (2008).
Buss, C. et al. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl Acad. Sci. USA 109, E1312–1319 (2012).
Gorgolewski, K. J. et al. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 9, 8 (2015).
Lebel, C. et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352 (2012).
Short, S. J. et al. Population variation in neuroendocrine activity is associated with behavioral inhibition and hemispheric brain structure in young rhesus monkeys. Psychoneuroendocrinology 47, 56–67 (2014).
van der Werff, S. J. et al. Widespread reductions of white matter integrity in patients with long-term remission of Cushing’s disease. Neuroimage Clin. 4, 659–667 (2014).
Pires, P. et al. White matter alterations in the brains of patients with active, remitted, and cured cushing syndrome: a DTI study. Am. J. Neuroradiol. 36, 1043–1048 (2015).
Magalhaes, R. et al. White matter changes in microstructure associated with a maladaptive response to stress in rats. Transl. Psychiatry 7, e1009 (2017).
Lebel, C., Walker, L., Leemans, A., Phillips, L. & Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055 (2008).
Jensen, S. K. G. et al. Associations between prenatal, childhood, and adolescent stress and variations in white-matter properties in young men. Neuroimage 182, 389–397 (2018).
Acknowledgements
The work was funded by the Danish Council for Independent Research (grant no.r: 09-071546), Gangstedfonden, Ville Heises Legat, Hvidovre Hospital’s Research Fund and the Lundbeck Foundation, and the Centre of Excellence grant to The Centre for Integrated Molecular Brain Imaging. H.R.S. holds a 5-year professorship in precision medicine at the Faculty of Health Sciences and Medicine, University of Copenhagen, which is sponsored by the Lundbeck Foundation (grant no. R186-2015-2138).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: M.V., W.F.C.B., S.K.H., O.B.P., A.P.B., P.U., H.R.S. and K.S.M. Performed the experiments: M.V. and S.K.H. Evaluated the data: M.V., W.F.C.B., S.K.H., C.G.M. and K.S.M. Analysed the data: M.V., W.F.C.B. and K.S.M. Contributed to writing the paper: M.V., W.F.C.B., S.K.H., O.B.P., A.P.B., P.U., H.R.S. and K.S.M.
Corresponding author
Ethics declarations
Competing interests
H.R.S. has received honoraria as speaker from Sanofi Genzyme, Denmark and Novartis, Denmark, as consultant from Sanofi Genzyme, Denmark and as senior editor (NeuroImage) from Elsevier Publishers, Amsterdam, The Netherlands. He has received royalties as book editor from Springer Publishers, Stuttgart, Germany. The other authors reported no competing interests.
Patient consent
Participants and their parents received thorough information regarding the project and written consent was obtained from all parents before study initiation. The study was approved by the Committee on Biomedical Research Ethics for the Capital Region of Denmark (protocol no. H-KF-01-131/03) and was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 3 has been corrected.
Supplementary information
Rights and permissions
About this article
Cite this article
Vestergaard, M., Baaré, W.F.C., Holm, S.K. et al. Glucocorticoid treatment for non-cerebral diseases in children and adolescents is associated with differences in uncinate fasciculus microstructure. Pediatr Res 91, 879–887 (2022). https://doi.org/10.1038/s41390-021-01394-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01394-w