Abstract
Background
In the developing brain, the death of immature oligodendrocytes (OLs) has been proposed to explain a developmental window for vulnerability to white matter injury (WMI). However, in neonatal mice, chronic sublethal intermittent hypoxia (IH) recapitulates the phenotype of diffuse WMI without affecting cellular viability. This work determines whether, in neonatal mice, a developmental window of WMI vulnerability exists in the absence of OLs lineage cellular death.
Methods
Neonatal mice were exposed to cell-nonlethal early or late IH stress. The presence or absence of WMI phenotype in their adulthood was defined by the extent of sensorimotor deficit and diffuse cerebral hypomyelination. A separate cohort of mice was examined for markers of cellular degeneration and OLs maturation.
Results
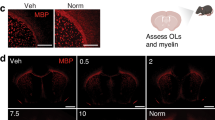
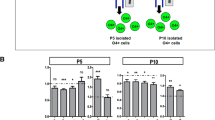
Compared to normoxic littermates, only mice exposed to early IH stress demonstrated arrested OLs maturation, diffuse cerebral hypomyelination, and sensorimotor deficit. No cellular death associated with IH was detected.
Conclusions
Neonatal sublethal IH recapitulates the phenotype of diffuse WMI only when IH stress coincides with the developmental stage of primary white matter myelination. This signifies a contribution of cell-nonlethal mechanisms in defining the developmental window of vulnerability to diffuse WMI.
Impact
-
The key message of our work is that the developmental window of vulnerability to the WMI driven by intermittent hypoxemia exists even in the absence of excessive OLs and other cells death.
-
This is an important finding because the existence of the developmental window of vulnerability to WMI has been explained by a lethal-selective sensitivity of immature OLs to hypoxic and ischemic stress, which coincided with their differentiation.
-
Thus, our study expands mechanistic explanation of a developmental window of sensitivity to WMI by showing the existence of cell-nonlethal pathways responsible for this biological phenomenon.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wilson-Costello, D., Fridedman, H., Minich, N., Fanaroff, A. & Hack, M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 115, 997–1003 (2005).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349 (2017).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440 (2011).
Back, S. A. et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 21, 1302–1312 (2001).
Rakic, S. & Zecevic, N. Early oligodendrocyte precursor cells in the human fetal telencephalon. Glia 41, 117–127 (2003).
Back, S. A. & Volpe, J. J. Cellular and molecular pathogenesis of periventricular white matter injury. MRDD Res. Rev. 3, 96–107 (1997).
Juliano, C. et al. Mild intermittent hypoxemia in neonatal mice causes permanent neurofunctional deficit and white matter hypomyelination. Exp. Neurol. 264, 33–42 (2015).
Niatsetskaya, Z. et al. Cyclophilin D–dependent oligodendrocyte mitochondrial ion leak contributes to neonatal white matter injury. J. Clin. Investig. 130, 5536–5550 (2020).
Poets, C. F. et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA J. Am. Med. Assoc. 314, 595–603 (2015).
Di Fiore, J. M. et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157, 69–73 (2010).
Dean, J. M. et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev. Neurosci. 33, 251–260 (2011).
Verity, A. N. & Campagnoni, A. T. Regional expression of myelin protein genes in the developing mouse brain: in situ hybridization studies. J. Neurosci. Res. 21, 238–248 (1988).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Haynes, R. L., Billiards, S. S., Borenstein, N. S., Volpe, J. J. & Kinney, H. C. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr. Res 63, 656–661 (2008).
Billiards, S. S. et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–163 (2008).
Verney, C. et al. Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. J. Neuropathol. Exp. Neurol. 71, 251–264 (2012).
Craig, A. et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp. Neurol. 181, 231–240 (2003).
Back, S. A. et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 22, 455–463 (2002).
Fern, R. & Moller, T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J. Neurosci. 20, 34–42 (2000).
Baud, O. et al. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 24, 1531–1540 (2004).
Buser, J. et al. Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J. Cereb. Blood Flow. Metab. 30, 1053–1065 (2010).
Tawk, M. et al. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J. Neurosci. 31, 3729–3742 (2011).
Fancy, S. P. et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 (2009).
Yue-Ying, P. et al. Altered Wnt signaling pathway in cognitive impairment caused by chronic intermittent hypoxia: focus on glycogen synthase kinase-3β and β-catenin. Chin. Med. J. 129, 838–845 (2016).
Scafidi, J. et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506, 230–234 (2014).
Van Steenwinckel, J. et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 142, 3806–3833 (2019).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Acknowledgements
This work was supported by NIH grants NS099109 and in part by NS100850 (V.S.T.). Image processing for this work was performed in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, supported by NIH grant P30 CA013696 (National Cancer Institute).
Author information
Authors and Affiliations
Contributions
S.A.S., V.I.R., and V.S.T. made substantial contributions to conception and design. S.A.S. and Z.V.N. made substantial contributions to data acquisition. All authors contributed to the analysis and interpretation of data, drafting/revising the intellectual content. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sosunov, S.A., Niatsetskaya, Z.V., Stepanova, A.A. et al. Developmental window of vulnerability to white matter injury driven by sublethal intermittent hypoxemia. Pediatr Res 91, 1383–1390 (2022). https://doi.org/10.1038/s41390-021-01555-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01555-x
This article is cited by
-
Minimum effective dose of clemastine in a mouse model of preterm white matter injury
Pediatric Research (2024)