Abstract
Background
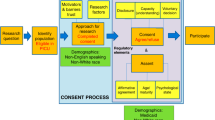
Obtaining informed consent for clinical research in the pediatric emergency department (ED) is challenging. Our objective was to understand the factors that influence parental consent for ED studies.
Methods
This was a cross-sectional survey assessing parents’ willingness to enroll their children into an ED research study. Parents reporting a willingness to enroll in ED studies were presented with two hypothetical scenarios, a low-risk and a high-risk study, and then asked about decision influencers affecting consent. Parents expressing a lack of willingness to enroll were asked which decision influencers impacted their consent decision.
Results
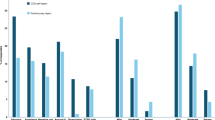
Among 118 parents, 90 (76%) stated they would be willing to enroll their child into an ED study; of these, 86 (96%) would consent for a low-risk study and 54 (60%) would consent for a high-risk study. Caucasian parents, and those with previous research exposure, were more likely to report willingness to participate. Those who would consent to the high-risk study cited “benefits that research would provide to future children” most strongly influenced their decision to agree.
Conclusions
ED investigators should highlight the benefits for future children and inquire about parents’ previous exposure to research to enhance ED research enrollment. Barriers to consent in non-Caucasian families should be further investigated.
Impact
-
Obtaining consent for pediatric emergency research is challenging and this study identified factors influencing parental consent for research in EDs.
-
Benefits for future children and parents’ previous research experience were two of the most influential factors in parents’ willingness to consent to ED research studies.
-
These findings will help to improve enrollment in ED research studies and better our understanding of how to promote the health and well-being of pediatric patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abernethy, L. E., Paulsen, E. L., Monuteaux, M. C., Berry, M. P. & Neuman, M. I. Parental perceptions of clinical research in the pediatric emergency department. Pediatr. Emerg. Care 29, 897–902 (2013).
Bourgeois, F. T. et al. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics 130, 285–292 (2012).
Morris, M. C., Besner, D., Vazquez, H., Nelson, R. M. & Fischbach, R. L. Parental opinions about clinical research. J. Pediatr. 151, 532–537 (2007).
Sammons, H. M., Atkinson, M., Choonara, I. & Stephenson, T. What motivates British parents to consent for research? A questionnaire study. BMC Pediatr. 7, 12 (2007).
Wendler, D., Abdoler, E., Wiener, L. & Grady, C. Views of adolescents and parents on pediatric research without the potential for clinical benefit. Pediatrics 130, 692–699 (2012).
Caldwell, P. H. Y., Butow, P. N. & Craig, J. C. Parents’ attitudes to children’s participation in randomized controlled trials. J. Pediatr. 142, 554–559 (2003).
Stenson, B. J., Becher, J. C. & McIntosh, N. Neonatal research: the parental perspective. Arch. Dis. Child. Fetal Neonatal Ed. 89, F321–F324 (2004).
Ruccione, K., Kramer, R. F., Moore, I. K. & Perin, G. Informed consent for treatment of childhood cancer: factors affecting parents’ decision making. J. Pediatr. Oncol. Nurs. 8, 112–121 (1991).
van Stuijvenberg, M. et al. Informed consent, parental awareness, and reasons for participating in a randomised controlled study. Arch. Dis. Child. 79, 120–125 (1998).
Eder, M. L., Yamokoski, A. D., Wittmann, P. W. & Kodish, E. D. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics 119, e849–e859 (2007).
Natale, J. E. et al. Racial and ethnic disparities in parental refusal of consent in a large, multisite pediatric critical care clinical trial. J. Pediatr. 184, 204–208 (2017).
Chamberlain, J. M. et al. Perceived challenges to obtaining informed consent for a time‐sensitive emergency department study of pediatric status epilepticus: results of two focus groups. Acad. Emerg. Med. 16, 763–770 (2009).
Heilbrunn, B. R. et al. Reducing anxiety in the pediatric emergency department: a comparative trial. J. Emerg. Med. 47, 623–631 (2014).
Holm, L. & Fitzmaurice, L. Factors influencing parent anxiety levels in a pediatric emergency department waiting area. Pediatr. Res. 56, 672 (2004).
Woolfall, K. et al. Doing challenging research studies in a patient-centred way: a qualitative study to inform a randomised controlled trial in the paediatric emergency care setting. BMJ Open 4, e005045 (2014).
Woolfall, K. et al. Fifteen-minute consultation: an evidence-based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Arch. Dis. Child. Educ. Pract. Ed. 101, 49–53 (2016).
Rabow, M. W., Hauser, J. M. & Adams, J. Supporting family caregivers at the end of life: they don’t know what they don’t know. JAMA 291, 483–491 (2004).
Greenberg, R. G. et al. Parents’ perceived obstacles to pediatric clinical trial participation: findings from the clinical trials transformation initiative. Contemp. Clin. Trials Commun. 9, 33–39 (2018).
Molyneux, S. et al. ‘The words will pass with the blowing wind’: staff and parent views of the deferred consent process, with prior assent, used in an emergency fluids trial in two African hospitals. PLoS ONE 8, e54894 (2013).
Glickman, S. W. et al. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann. Emerg. Med. 51, 775–780 (2008).
Kelly, M. L., Ackerman, P. D. & Ross, L. F. The participation of minorities in published pediatric research. J. Natl Med. Assoc. 97, 777 (2005).
Stiles, P. G., Epstein, M., Poythress, N. & Edens, J. F. Protecting people who decline to participate in research: an example from a prison setting. IRB-Ethics Hum. Res. 34, 15 (2012).
Kim, H.-Y. Statistical notes for clinical researchers: chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 42, 152–155 (2017).
Haddad, A. End-of-life decisions: the family’s role. RN 67, 25–28 (2004).
Allmark, P. & Mason, S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J. Med. Ethics 32, 439–443 (2006).
Nelson, D. K. et al. Obtaining consent from both parents for pediatric research: what does “reasonably available” mean? Pediatrics 131, e223–e229 (2013).
Brandt, A. M. Racism and research: the case of the Tuskegee Syphilis Study. Hastings Cent. Rep. 8, 21–29 (1978).
Freimuth, V. S. et al. African Americans’ views on research and the Tuskegee Syphilis Study. Soc. Sci. Med. 52, 797–808 (2001).
Lebet, R., Fineman, L. D., Faustino, E. V. S. & Curley, M. A. Q. Asking for parents’ permission to enroll their child into a clinical trial: best practices. Am. J. Crit. Care 22, 351–356 (2013).
Sheidow, A. J., Henry, D. B., Tolan, P. H. & Strachan, M. K. The role of stress exposure and family functioning in internalizing outcomes of urban families. J. Child Fam. Stud. 23, 1351–1365 (2014).
Acknowledgements
We would like to thank Dr. Daniela Santos for translating the surveys into Spanish and for enrolling patients in the study. We would also like to thank Marissa Garcia, Tate Closson-Niese, Amira Herstic, and Kayla Bell for enrolling patients into this study. Additionally, this study could not have been conducted without the help of Mimi Goodwin and Kathleen Grice. There was no external funding for this manuscript and all authors have indicated they have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Contributions
R.L.M. conceptualized and designed the study and data collection instruments, collected data, and initiated and revised the manuscript. R.D.M. and R.D.C. conceptualized and designed the study and data collection instruments, supervised the study, reviewed the manuscript for important intellectual content, and revised the manuscript. R.D.M. provided final approval of the version to be published. L.B. conceptualized the study and reviewed and revised the manuscript. L.P. and J.L. made substantial contributions to the analysis and interpretation of data and helped to revise critical manuscript content. All authors are in agreement with the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Consent was provided through the completion of the survey.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Miller, R.L., Comstock, R.D., Pierpoint, L. et al. Facilitators and barriers for parental consent to pediatric emergency research. Pediatr Res 91, 1156–1162 (2022). https://doi.org/10.1038/s41390-021-01600-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01600-9