Abstract
Background
Necrotizing enterocolitis (NEC), a severe gut disorder in preterm infants, is difficult to predict due to poor specificity and sensitivity of clinical signs and biomarkers. Using preterm piglets as a model, we hypothesized that early development of NEC affects blood gene expression, potentially related to early systemic immune responses.
Methods
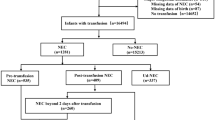
A retrospective analysis of clinical, tissue, and blood data was performed on 129 formula-fed piglets with NEC diagnosis at necropsy on day 5. Subgroups of NEC (n = 20) and control piglets (CON, n = 19) were analyzed for whole-blood transcriptome.
Results
Preterm piglets had variable NEC lesions, especially in the colon region, without severe clinical signs (e.g. normal growth, activity, hematology, digestion, few piglets with bloody stools). Transcriptome analysis showed 344 differentially expressed genes (DEGs) between NEC and CON piglets. Validation experiment showed that AOAH, ARG2, FKBP5, PAK2, and STAT3 were among the genes affected by severe lesions on day 5, when analyzed in whole blood and in dried blood spots (DBS).
Conclusion
Whole-blood gene expressions may be affected in preterm pigs before clinical signs of NEC get severe. Blood gene expression analysis, potentially using DBS samples, is a novel tool to help identify new early biomarkers of NEC.
Impact
-
Preterm pig model was used to investigate if blood transcriptomics could be used to identify new early blood biomarkers of NEC progression.
-
Whole-blood transcriptome revealed upregulation of target genes in NEC cases when clinical symptoms are subtle, and mainly colon regions were affected.
-
Differential NEC-associated gene expressions could be detected also in dried blood spots, potentially allowing easy collection of small blood volumes in infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All sequencing and processed data are deposited in the Gene Expression Omnibus (GEO) with accession number GSE166152.
References
Patel, R. M. et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N. Engl. J. Med. 372, 331–340 (2015).
D’Angelo, G. et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital. J. Pediatr. 44, 84 (2018).
Juhl, S. M., Gregersen, R., Lange, T. & Greisen, G. Incidence and risk of necrotizing enterocolitis in Denmark from 1994–2014. PLoS ONE 14, e0219268 (2019).
Luig, M., Lui, K., Nsw & Group, A. N. Epidemiology of necrotizing enterocolitis—Part I: Changing regional trends in extremely preterm infants over 14 years. J. Paediatr. Child Health 41, 169–173 (2005).
Ng, P. C., Ma, T. P. & Lam, H. S. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch. Dis. Child Fetal Neonatal Ed. 100, F448–F452 (2015).
Ng, E. W. et al. Gut-associated biomarkers L-FABP, I-FABP, and TFF3 and LIT score for diagnosis of surgical necrotizing enterocolitis in preterm infants. Ann. Surg. 258, 1111–1118 (2013).
Luo, J., Li, H. P., Xu, F., Wu, B. Q. & Lin, H. C. Early diagnosis of necrotizing enterocolitis by plasma RELMbeta and thrombocytopenia in preterm infants: a pilot study. Pediatr. Neonatol. 60, 447–452 (2019).
Agakidou, E., Agakidis, C., Gika, H. & Sarafidis, K. Emerging biomarkers for prediction and early diagnosis of necrotizing enterocolitis in the era of metabolomics and proteomics. Front. Pediatr. 8, 602255 (2020).
Morrow, A. L. et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1, 13 (2013).
Leaphart, C. L. et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 (2007).
Sodhi, C. P. et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 (2010).
Egan, C. E. et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J. Clin. Invest. 126, 495–508 (2016).
Ng, P. C. et al. Plasma miR-1290 is a novel and specific biomarker for early diagnosis of necrotizing enterocolitis-biomarker discovery with prospective cohort evaluation. J. Pediatr. 205, 83–90. e10 (2019).
Grauholm, J. et al. Gene expression profiling of archived dried blood spot samples from the Danish Neonatal Screening Biobank. Mol. Genet. Metab. 116, 119–124 (2015).
Sangild, P. T. et al. Invited review: the preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 91, 4713–4729 (2013).
Ren, S. et al. Neonatal gut and immune maturation is determined more by postnatal age than by postconceptional age in moderately preterm pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G855–G867 (2018).
Baek, O. et al. Diet modulates the high sensitivity to systemic infection in newborn preterm pigs. Front. Immunol. 11, 1019 (2020).
Cao, M. et al. Physical activity and gastric residuals as biomarkers for region-specific NEC lesions in preterm neonates. Neonatology 110, 241–247 (2016).
Sun, J. et al. Necrotizing enterocolitis is associated with acute brain responses in preterm pigs. J. Neuroinflamm. 15, 180 (2018).
Nguyen, D. N. et al. Prenatal intra-amniotic endotoxin induces fetal gut and lung immune responses and postnatal systemic inflammation in preterm pigs. Am. J. Pathol. 188, 2629–2643 (2018).
Holgersen, K. et al. Supplemental insulin-like growth factor-1 and necrotizing enterocolitis in preterm pigs. Front. Pediatr. 8, 602047 (2020).
Cao, M. et al. Physical activity level is impaired and diet dependent in preterm newborn pigs. Pediatr. Res. 78, 137–144 (2015).
Thymann, T. et al. Formula-feeding reduces lactose digestive capacity in neonatal pigs. Br. J. Nutr. 95, 1075–1081 (2006).
Stoy, A. C. F. et al. Increased intestinal inflammation and digestive dysfunction in preterm pigs with severe necrotizing enterocolitis. Neonatology 111, 289–296 (2017).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ye, J. et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134 (2012).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
van Dam, S., Vosa, U., van der Graaf, A., Franke, L. & de Magalhaes, J. P. Gene co-expression analysis for functional classification and gene-disease predictions. Brief. Bioinformatics 19, 575–592 (2018).
Liu, T. et al. Quantitative proteomic analysis of intracerebral hemorrhage in rats with a focus on brain energy metabolism. Brain Behav. 8, e01130 (2018).
Tsuchida, C. et al. Expression of REG family genes in human inflammatory bowel diseases and its regulation. Biochem. Biophys. Rep. 12, 198–205 (2017).
Bouzid, D. et al. Association of ZAP70 and PTPN6, but Not BANK1 or CLEC2D, with inflammatory bowel disease in the Tunisian population. Genet. Test. Mol. Biomark. 17, 321–326 (2013).
Boros, E. et al. Elevated expression of AXL may contribute to the epithelial-to-mesenchymal transition in inflammatory bowel disease patients. Mediat. Inflamm. 2018, 3241406 (2018).
Lyons, J. et al. Integrated in vivo multiomics analysis identifies p21-activated kinase signaling as a driver of colitis. Sci. Signal. 11, eaan3580 (2018).
Li, Y. et al. Bovine colostrum before or after formula feeding improves systemic immune protection and gut function in newborn preterm pigs. Front. Immunol. 10, 3062 (2019).
Oulmaati, A. et al. Risk factors of mild rectal bleeding in very low birth weight infants: a case control study. BMC Pediatr. 13, 196 (2013).
Maayan-Metzger, A., Ghanem, N., Mazkereth, R. & Kuint, J. Characteristics of neonates with isolated rectal bleeding. Arch. Dis. Child Fetal Neonatal Ed. 89, F68–F70 (2004).
Stoy, A. C. et al. Bovine colostrum improves intestinal function following formula-induced gut inflammation in preterm pigs. Clin. Nutr. 33, 322–329 (2014).
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Ren S, et al. Sub-clinical necrotizing enterocolitis-induced systemic immune suppression in neonatal preterm pigs. Am J Physiol Gastrointest Liver Physiol. (2021)
Ren, S., Pan, X., Gao, F., Sangild, P. T. & Nguyen, D. N. Prenatal inflammation suppresses blood Th1 polarization and gene clusters related to cellular energy metabolism in preterm newborns. FASEB J. 34, 2896–2911 (2020).
Nino, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Robinson, J. L. et al. Prematurity reduces citrulline-arginine-nitric oxide production and precedes the onset of necrotizing enterocolitis in piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G638–G649 (2018).
Shah, P. S., Shah, V. S. & Kelly, L. E. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 4, CD004339 (2017).
Maltese, P. et al. Glucocorticoid resistance in Crohn’s disease and ulcerative colitis: an association study investigating GR and FKBP5 gene polymorphisms. Pharmacogenomics J. 12, 432–438 (2012).
Wochnik, G. M. et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 280, 4609–4616 (2005).
Sangild, P. T. et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130, 1776–1792 (2006).
Siggers, J. et al. Transition from parenteral to enteral nutrition induces immediate diet-dependent gut histological and immunological responses in preterm neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G435–G445 (2011).
Gauffin, F., Nordgren, A., Barbany, G., Gustafsson, B. & Karlsson, H. Quantitation of RNA decay in dried blood spots during 20 years of storage. Clin. Chem. Lab. Med. 47, 1467–1469 (2009).
Acknowledgements
We thank Thomas Thymann, Anders Brunse, Duc Ninh Nguyen, Jing Sun, Kristine Holgersen, Jane Povlsen, Elin Skytte, Kristina Møller (University of Copenhagen), and Jonas Bybjerg-Grauholma (Statens Serum Institut, Denmark) for their support to animal procedures and laboratory analyses. This work was supported by the Innovation Foundation Denmark NEOCOL project (to P.T.S.) and the Agricultural Science and Technology Innovation Program (ASTIP) of China (to F.G.).
Author information
Authors and Affiliations
Contributions
X.P. analyzed and interpreted the transcriptome data, and was the major contributor in writing the manuscript. T.M., S.R., and D.N.N. performed animal experiment and analysis. D.N.N., R.L.S., F.G., and P.T.S. took part in the main study design and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Pan, X., Muk, T., Ren, S. et al. Blood transcriptomic markers of necrotizing enterocolitis in preterm pigs. Pediatr Res 91, 1113–1120 (2022). https://doi.org/10.1038/s41390-021-01605-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01605-4
This article is cited by
-
Multi-strain probiotic administration decreases necrotizing enterocolitis severity and alters the epigenetic profile in mice
Pediatric Research (2025)
-
Neonatal enteral antibiotics reduce gut inflammation and delay systemic immune development in preterm pigs
Pediatric Research (2025)
-
Data-independent acquisition-based blood proteomics unveils predictive biomarkers for neonatal necrotizing enterocolitis
Analytical and Bioanalytical Chemistry (2025)
-
Identifying immune signatures of sepsis to increase diagnostic accuracy in very preterm babies
Nature Communications (2024)
-
Brain lipidomics and neurodevelopmental outcomes in intrauterine growth restricted piglets fed dairy or vegetable fat diets
Scientific Reports (2022)