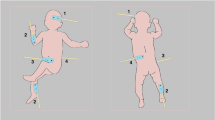
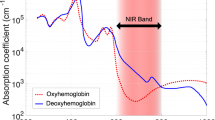
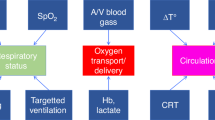
Abstract
Perioperative applications of near-infrared spectroscopy (NIRS) to monitor regional tissue oxygenation and perfusion in cardiac and noncardiac surgery are of increasing interest in neonatal care. Complex neonatal surgery can impair adequate oxygen delivery and tissue oxygen consumption and increase the risk of neurodevelopmental delay. Coupled with conventional techniques, NIRS monitoring may enable targeted hemodynamic management of the circulation in both cardiac and noncardiac surgical procedures. In this narrative review, we discuss the application of perioperative NIRS in specific neonatal interventions, including surgical intervention for congenital heart defects, definitive closure of the patent ductus arteriosus, neurological and gastrointestinal disorders, and use of extracorporeal membrane oxygenation. We identified areas for future research within disease-specific indications and offer a roadmap to aid in developing evidence-based targeted diagnostic and management strategies in neonates.
Impact
There is growing recognition that perioperative NIRS monitoring, used in conjunction with conventional monitoring, may provide critical hemodynamic information that either complements clinical impressions or delivers novel physiologic insight into the neonatal circulatory and perfusion pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stolwijk, L. J. et al. Neurodevelopmental outcomes after neonatal surgery for major noncardiac anomalies. Pediatrics 137, e20151728 (2016).
Stolwijk, L. J. et al. Neonatal surgery for noncardiac congenital anomalies: neonates at risk of brain injury. J. Pediatr. 182, 335–341.e331 (2017).
Sakamoto, T. Current status of brain protection during surgery for congenital cardiac defect. Gen. Thorac. Cardiovasc. Surg. 64, 72–81 (2015).
Hansen, M. L. et al. The clinical effects of cerebral near-infrared spectroscopy monitoring (NIRS) versus no monitoring: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst. Rev. 10, 111 (2021).
Dix, L. M. L., Van Bel, F., Baerts, W. & Lemmers, P. M. A. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr. Res. 74, 557–563 (2013).
Schneider, A. et al. Comparison of four near-infrared spectroscopy devices shows that they are only suitable for monitoring cerebral oxygenation trends in preterm infants. Acta Paediatr. 103, 934–938 (2014).
Vesoulis, Z. A., Mintzer, J. P. & Chock, V. Y. Neonatal NIRS monitoring: recommendations for data capture and review of analytics. J. Perinatol. 41, 675–688 (2021).
Nagaraj, U. D. et al. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J. Pediatr. 167, 1018–1024 (2015).
Cheng, H. H. et al. Abnormalities in cerebral hemodynamics and changes with surgical intervention in neonates with congenital heart disease. J. Thorac. Cardiovasc. Surg. 159, 2012–2021 (2020).
Beck, J. et al. Monitoring cerebral and renal oxygenation status during neonatal digestive surgeries using near infrared spectroscopy. Front. Pediatr. 5, 140 (2017).
Mebius, M. J. et al. Onset of brain injury in infants with prenatally diagnosed congenital heart disease. PLoS ONE 15, e0230414 (2020).
Mebius, M. J. et al. Cerebral oxygen saturation during the first 72h after birth in infants diagnosed prenatally with congenital heart disease. Early Hum. Dev. 103, 199–203 (2016).
Zaleski, K. L. & Kussman, B. D. Near-infrared spectroscopy in pediatric congenital heart disease. J. Cardiothorac. Vasc. Anesth. 34, 489–500 (2020).
Koch, H. W. & Hansen, T. G. Perioperative use of cerebral and renal near-infrared spectroscopy in neonates: a 24-h observational study. Pediatr. Anaesth. 26, 190–198 (2015).
Cruz, S. M. et al. A novel multimodal computational system using near-infrared spectroscopy to monitor cerebral oxygenation during assisted ventilation in CDH patients. J. Pediatr. Surg. 51, 38–43 (2016).
Sood, E. D., Benzaquen, J. S., Davies, R. R., Woodford, E. & Pizarro, C. Predictive value of perioperative near-infrared spectroscopy for neurodevelopmental outcomes after cardiac surgery in infancy. J. Thorac. Cardiovasc. Surg. 145, 438–445.e431 (2013). discussion 444–435.
Petit, C. J. et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulatio 119, 709–716 (2009).
Lynch, J. M. et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 148, 2181–2188 (2014).
Lynch, J. M. et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 156, 1657–1664 (2018).
Bravo, Md. C. et al. Acute effects of levosimendan on cerebral and systemic perfusion and oxygenation in newborns: an observational study. Neonatology 99, 217–223 (2011).
Pellicer, A. et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr. Res. 73, 95–103 (2012).
Lejus, C. et al. A retrospective study about cerebral near-infrared spectroscopy monitoring during paediatric cardiac surgery and intra-operative patient blood management. Anaesth. Crit. Care Pain Med. 34, 259–263 (2015).
Menke, J. & Möller, G. Cerebral near-infrared spectroscopy correlates to vital parameters during cardiopulmonary bypass surgery in children. Pediatr. Cardiol. 35, 155–163 (2013).
Hoffman, G. M., Brosig, C. L., Mussatto, K. A., Tweddell, J. S. & Ghanayem, N. S. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J. Thorac. Cardiovasc. Surg. 146, 1153–1164 (2013).
Dent, C. L. et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J. Thorac. Cardiovasc. Surg. 130, 1523–1530 (2005).
Claessens, N. H. P. et al. Postoperative cerebral oxygenation was not associated with new brain injury in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 158, 867–877.e861 (2019).
Aly, S. A. et al. Cerebral tissue oxygenation index and lactate at 24 h postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit. Heart Dis. 12, 188–195 (2016).
Zulueta, J. L., Vida, V. L., Perisinotto, E., Pittarello, D. & Stellin, G. The role of intraoperative regional oxygen saturation using near infrared spectroscopy in the prediction of low output syndrome after pediatric heart surgery. J. Card. Surg. 28, 446–452 (2013).
Backes, C. H. et al. Percutaneous closure of the patent ductus arteriosus in very low weight infants: considerations following US Food and Drug Administration approval of a novel device. J. Pediatr. 213, 218–221 (2019).
Hamrick, S. E. G. et al. Patent ductus arteriosus of the preterm infant. Pediatrics 146, e20201209 (2020).
El-Khuffash, A., Levy, P. T., Gorenflo, M. & Frantz, I. D. The definition of a hemodynamically significant ductus arteriosus. Pediatr. Res. 85, 740–741 (2019).
Kabra, N. S. et al. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. J. Pediatr. 150, 229–234.e221 (2007).
Chorne, N., Leonard, C., Piecuch, R. & Clyman, R. I. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics 119, 1165–1174 (2007).
Weisz, D. E. et al. Association of patent ductus arteriosus ligation with death or neurodevelopmental impairment among extremely preterm infants. JAMA Pediatr. 171, 443–449 (2017).
Bravo, M. C., Ybarra, M., Madero, R. & Pellicer, A. Childhood neurodevelopmental outcome in low birth weight infants with post-ligation cardiac syndrome after ductus arteriosus closure. Front. Physiol. 10, 718 (2019).
Heuchan, A. M., Hunter, L. & Young, D. Outcomes following the surgical ligation of the patent ductus arteriosus in premature infants in Scotland. Arch. Dis. Child Fetal Neonatal Ed. 97, F39–F44 (2012).
Wickremasinghe, A. C. et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J. Pediatr. 161, 1065–1072 (2012).
Weisz, D. E., More, K., McNamara, P. J. & Shah, P. S. PDA ligation and health outcomes: a meta-analysis. Pediatrics 133, e1024–e1046 (2014).
Ito, S. et al. Surgical ligation for patent ductus arteriosus in extremely premature infants: strategy to reduce their risk of neurodevelopmental impairment. Tohoku J. Exp. Med. 240, 7–13 (2016).
Henry, B. M. et al. Incidence, risk factors, and comorbidities of vocal cord paralysis after surgical closure of a patent ductus arteriosus: a meta-analysis. Pediatr. Cardiol. 40, 116–125 (2019).
Stankowski, T. et al. Surgical closure of patent ductus arteriosus in extremely low birth weight infants weighing less than 750 grams. Kardiol. Pol. 76, 750–754 (2018).
Lehenbauer, D. G. et al. Surgical closure of patent ductus arteriosus in premature neonates weighing less than 1,000 grams: contemporary outcomes. World J. Pediatr. Congenit. Heart Surg. 9, 419–423 (2018).
Foster, M. et al. Short-term complications associated with surgical ligation of patent ductus arteriosus in elbw infants: a 25-year cohort study. Am. J. Perinatol. 38, 477–481 (2019).
Lemmers, P. M., Molenschot, M. C., Evens, J., Toet, M. C. & van Bel, F. Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch. Dis. Child Fetal Neonatal Ed. 95, F429–F434 (2010).
Lemmers, P. M. et al. Patent ductus arteriosus and brain volume. Pediatrics 137, e20153090 (2016).
Zaramella, P. et al. Surgical closure of patent ductus arteriosus reduces the cerebral tissue oxygenation index in preterm infants: a near-infrared spectroscopy and Doppler study. Pediatr. Int 48, 305–312 (2006).
Hüning, B. M., Asfour, B., König, S., Hess, N. & Roll, C. Cerebral blood volume changes during closure by surgery of patent ductus arteriosus. Arch. Dis. Child Fetal Neonatal Ed. 93, F261–F264 (2008).
Vanderhaegen, J. et al. Surgical closure of the patent ductus arteriosus and its effect on the cerebral tissue oxygenation. Acta Paediatr. 97, 1640–1644 (2008).
Chock, V. Y., Ramamoorthy, C. & Van Meurs, K. P. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J. Pediatr. 160, 936–942 (2012).
Chock, V. Y., Ramamoorthy, C. & Van Meurs, K. P. Cerebral oxygenation during different treatment strategies for a patent ductus arteriosus. Neonatology 100, 233–240 (2011).
Hou, X. et al. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near-infrared spectroscopy. Physiol. Meas. 28, 1251–1265 (2007).
Cohen, E. et al. Reduction in cerebral oxygenation due to patent ductus arteriosus is pronounced in small-for-gestational-age neonates. Neonatology 111, 126–132 (2016).
Villamor-Martinez, E., Kilani, M. A., Degraeuwe, P. L., Clyman, R. I. & Villamor, E. Intrauterine growth restriction and patent ductus arteriosus in very and extremely preterm infants: a systematic review and meta-analysis. Front. Endocrinol. 10, 58 (2019).
MacLaren, A. T. & Heuchan, A. M. Effect of surgical ligation of the patent ductus arteriosus on cerebral perfusion of premature infants in the postoperative period. Arch. Dis. Child Fetal Neonatal Ed. 101, F277 (2016).
Kooi, E. M. W. et al. Cerebrovascular autoregulation in preterm infants during and after surgical ligation of the ductus arteriosus, a comparison between two surgical approaches. Front. Pediatr. 8, 334 (2020).
Arora, R. et al. Preservation of the metabolic rate of oxygen in preterm infants during indomethacin therapy for closure of the ductus arteriosus. Pediatr. Res. 73, 713–718 (2013).
Lin, P.-Y., Hagan, K., Fenoglio, A., Grant, P. E. & Franceschini, M. A. Reduced cerebral blood flow and oxygen metabolism in extremely preterm neonates with low-grade germinal matrix- intraventricular hemorrhage. Sci. Rep. 16, 25903 (2016).
Teixeira, L. S., Shivananda, S. P., Stephens, D., Van Arsdell, G. & McNamara, P. J. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J. Perinatol. 28, 803–810 (2008).
McNamara, P. J., Stewart, L., Shivananda, S. P., Stephens, D. & Sehgal, A. Patent ductus arteriosus ligation is associated with impaired left ventricular systolic performance in premature infants weighing less than 1000 g. J. Thorac. Cardiovasc. Surg. 140, 150–157 (2010).
Noori, S., Friedlich, P., Seri, I. & Wong, P. Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J. Pediatr. 150, 597–602 (2007).
Harting, M. T. et al. Acute hemodynamic decompensation following patent ductus arteriosus ligation in premature infants. J. Invest. Surg. 21, 133–138 (2008).
Giesinger, R. E. et al. Impaired right ventricular function is associated with adverse outcome following hypoxic ischemic encephalopathy. Am. J. Respir. Crit. Care Med. 200, 1294–1305 (2019).
Halliday, M., Kavarana, M., Ebeling, M. & Kiger, J. Milrinone use for hemodynamic instability in patent ductus arteriosus ligation. J. Matern. Fetal Neonatal Med. 30, 529–533 (2017).
Ting, J. Y. et al. Predictors of respiratory instability in neonates undergoing patient ductus arteriosus ligation after the introduction of targeted milrinone treatment. J. Thorac. Cardiovasc. Surg. 152, 498–504 (2016).
Nealon, E. et al. Follow-up after percutaneous patent ductus arteriosus occlusion in lower weight infants. J. Pediatr. 212, 144–150.e143 (2019).
Ulrich, T. J. B., Hansen, T. P., Reid, K. J., Bingler, M. A. & Olsen, S. L. Post-ligation cardiac syndrome is associated with increased morbidity in preterm infants. J. Perinatol. 38, 537–542 (2018).
Lien, R., Hsu, K. H., Chu, J. J. & Chang, Y. S. Hemodynamic alterations recorded by electrical cardiometry during ligation of ductus arteriosus in preterm infants. Eur. J. Pediatr. 174, 543–550 (2015).
Gray, M. A. et al. Preoperative echocardiographic measures of left ventricular mechanics are associated with postoperative vasoactive support in preterm infants undergoing patent ductus arteriosus ligation. J. Thorac. Cardiovasc. Surg. 154, 2054–2059.e2051 (2017).
Rios, D. R., Bhattacharya, S., Levy, P. T. & McNamara, P. J. Circulatory insufficiency and hypotension related to the ductus arteriosus in neonates. Front. Pediatr. 6, 62 (2018).
Abdel-Bary, M. et al. Left ventricular dysfunction postsurgical patent ductus arteriosus ligation in children: predictor factors analysis. J. Cardiothorac. Surg. 14, 168 (2019).
Michel-Macias, C., Morales-Barquet, D. A., Martinez-Garcia, A. & Ibarra-Rios, D. Findings from somatic and cerebral near-infrared spectroscopy and echocardiographic monitoring during ductus arteriosus ligation: description of two cases and review of literature. Front. Pediatr. 8, 523 (2020).
Sathanandam, S. K. et al. Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc. Interv. 96, 1266–1276 (2020).
Adrianne Rahde Bischoff, M. D. et al. Percutaneous closure of patent ductus arteriosus in infants ≤1.5 kg: a meta-analysis. J. Pediatr. 230, 84–92.e14 (2021).
Shi, X., Hua, Y. & Li, Y. Transcatheter closure of patent ductus arteriosus in preterm ventilation-dependent neonates: a case series report. Medicine 99, e22528 (2020).
Serrano, R. M., Madison, M., Lorant, D., Hoyer, M. & Alexy, R. Comparison of ‘Post-Patent Ductus Arteriosus Ligation Syndrome’ in premature infants after surgical ligation vs. percutaneous closure. J. Perinatol. 40, 324–329 (2020).
Fraisse, A., Bautista-Rodriguez, C., Burmester, M., Lane, M. & Singh, Y. Transcatheter closure of patent ductus arteriosus in infants with weight under 1,500 grams. Front. Pediatr. 8, 558256 (2020).
O’Byrne, M. L. et al. Trends in transcatheter and operative closure of patent ductus arteriosus in neonatal intensive care units: analysis of data from the Pediatric Health Information Systems Database. Am. Heart J. 217, 121–130 (2019).
Kim, H. S. et al. Surgical versus percutaneous closure of pda in preterm infants: procedural charges and outcomes. J. Surg. Res. 243, 41–46 (2019).
Rodriguez Ogando, A. et al. Surgical ligation versus percutaneous closure of patent ductus arteriosus in very low-weight preterm infants: which are the real benefits of the percutaneous approach? Pediatr. Cardiol. 39, 398–410 (2018).
Norooz, F. et al. Decompressing posthaemorrhagic ventricular dilatation significantly improves regional cerebral oxygen saturation in preterm infants. Acta Paediatr. 104, 663–669 (2015).
Bembich, S., Cont, G., Bua, J., Paviotti, G. & Demarini, S. Cerebral hemodynamics during neonatal cerebrospinal fluid removal. Pediatr. Neurol. 94, 70–73 (2019).
Soul, J. S., Eichenwald, E., Walter, G., Volpe, J. J. & du Plessis, A. J. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr. Res. 55, 872–876 (2004).
van Alfen-van der Velden, A. A. et al. Cerebral hemodynamics and oxygenation after serial CSF drainage in infants with PHVD. Brain Dev. 29, 623–629 (2007).
Mahoney, L., Luyt, K., Harding, D. & Odd, D. Treatment for post-hemorrhagic ventricular dilatation: a multiple-treatment meta-analysis. Front. Pediatr. 8, 238 (2020).
Kochan, M. et al. Changes in cerebral oxygenation in preterm infants with progressive posthemorrhagic ventricular dilatation. Pediatr. Neurol. 73, 57–63 (2017).
McLachlan, P. J. et al. Investigating the effects of cerebrospinal fluid removal on cerebral blood flow and oxidative metabolism in infants with post-hemorrhagic ventricular dilatation. Pediatr. Res. 82, 634–641 (2017).
Rhee, C. J. et al. Neonatal cerebrovascular autoregulation. Pediatr. Res. 84, 602–610 (2018).
Chock, V. Y. et al. Cerebral oxygenation and autoregulation in preterm infants (Early NIRS Study). J. Pediatr. 227, 94–100.e101 (2020).
Cimatti, A. G. et al. Cerebral oxygenation and autoregulation in very preterm infants developing ivh during the transitional period: a pilot study. Front. Pediatr. 8, 381 (2020).
Åkerlund, C. A. et al. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a Center-TBI High-Resolution Group Study. PLoS ONE 15, e0243427 (2020).
Güiza, F. et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intens. Care Med. 41, 1067–1076 (2015).
Conforti, A. et al. Near infrared spectroscopy: experience on esophageal atresia infants. J. Pediatr. Surg. 49, 1064–1068 (2014).
Tytgat, S. H. A. J. et al. Neonatal brain oxygenation during thoracoscopic correction of esophageal atresia. Surg. Endosc. 30, 2811–2817 (2015).
Costerus, S., Vlot, J., van Rosmalen, J., Wijnen, R. & Weber, F. Effects of neonatal thoracoscopic surgery on tissue oxygenation: a pilot study on (neuro-) monitoring and outcomes. Eur. J. Pediatr. Surg. 29, 166–172 (2019).
Cruz, S. M. et al. A novel multimodal computational system using near-infrared spectroscopy predicts the need for ECMO initiation in neonates with congenital diaphragmatic hernia. J. Pediatr. Surg. 53, 152–158 (2018).
Muñoz, A. et al. Cerebral and renal oxygenation in infants undergoing laparoscopic gastrostomy tube placement. J. Surg. Res. 256, 83–89 (2020).
Stienstra, R. M. & McHoney, M. Near-infrared spectroscopy (NIRS) measured tissue oxygenation in neonates with gastroschisis: a pilot study. J. Matern. Fetal Neonatal Med. 18, 1–9 (2021).
McHoney, M. & Munro, F. Intestinal ischemia secondary to volvulus of gastroschisis within a silo: detection, confirmation and reversal of near infra-red spectroscopy detected O2 saturation. Pediatr. Surg. Int. 30, 1173–1176 (2014).
Cortez, J. et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 24, 574–582 (2011).
Patel, A. K. et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 15, 735–741 (2014).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinat. Med. 7, 199–206 (2014).
Schat, T. E. et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS ONE 11, e0154710 (2016).
van der Heide, M., Hulscher, J. B. F., Bos, A. F. & Kooi, E. M. W. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 90, 148–155 (2021).
Le Bouhellec, J. et al. Near-Infrared spectroscopy: a tool for diagnosing necrotizing enterocolitis at onset of symptoms in preterm neonates with acute gastrointestinal symptoms? Am. J. Perinatol. 38, e299–e308 (2021).
Palleri, E., Wackernagel, D., Wester, T. & Bartocci, M. Low splanchnic oxygenation and risk for necrotizing enterocolitis in extremely preterm newborns. J. Pediatr. Gastroenterol. Nutr. 71, 401–406 (2020).
Kuik, S. J. et al. Intestinal oxygenation and survival after surgery for necrotizing enterocolitis: an observational cohort study. Ann. Surg. https://doi.org/10.1097/SLA.000000000000391, 1–8 (2020).
Blau, J. et al. ransfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J. Pediatr. 158, 403–409 (2011).
Marin, T. & Moore, J. E. Mesenteric oxygenation changes associated with necrotizing enterocolitis and pneumoperitoneum after multiple blood transfusions: a case report. Adv. Neonatal Care 18, 121–127 (2018).
Baserga, M., Reich, B. & Braski, K. Abnormal splanchnic regional saturations in a preterm infant that developed necrotizing enterocolitis following a red blood cell transfusion. Adv. Neonatal Care 20, 401–405 (2020).
Kirpalani, H. et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N. Engl. J. Med. 383, 2639–2651 (2020).
Kalteren, W. S. et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am. J. Perinatol. 35, 1031–1037 (2018).
Kuik, S. J. et al. Preterm infants undergoing laparotomy for necrotizing enterocolitis or spontaneous intestinal perforation display evidence of impaired cerebrovascular autoregulation. Early Hum. Dev. 118, 25–31 (2018).
Schat, T. E. et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum. Dev. 131, 75–80 (2019).
Howarth, C. et al. Cerebral oxygenation in preterm infants with necrotizing enterocolitis. Pediatrics 146, 1–11 (2020).
Erdil, T. et al. Extracorporeal membrane oxygenation support in pediatrics. Ann. Cardiothorac. Surg. 8, 109–115 (2019).
ELSO Registry Report. International Summary January 2020. https://www.elso.org/Registry.aspx (2020).
Polito, A. et al. Eurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intens. Care Med. 39, 1594–1601 (2013).
Rais-Bahrami, K., Wagner, A. E., Coffman, C., Glass, P. & Short, B. L. Neurodevelopmental outcome in ECMO vs near-miss ECMO patients at 5 years of age. Clin. Pediatr. 39, 145–152 (2000).
McNally, H., Bennett, C. C., Elbourne, D. & Field, D. J. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics 117, e845–e854 (2006).
Schiller, R. M. et al. Neuropsychological follow-up after neonatal ECMO. Pediatrics 138, e20161313 (2016).
Leeuwen, L. et al. Risk factors of impaired neuropsychologic outcome in school-aged survivors of neonatal critical illness. Crit. Care Med. 46, 401–410 (2018).
Tsou, P.-Y., Garcia, A. V., Yiu, A., Vaidya, D. M. & Bembea, M. M. Association of cerebral oximetry with outcomes after extracorporeal membrane oxygenation. Neurocrit. Care. 33, 429–437 (2020).
Liem, K. D., Kollée, L. A., Klaessens, J. H., De Haan, A. F. & Oeseburg, B. Disturbance of cerebral oxygenation and hemodynamics related to the opening of the bypass bridge during veno-arterial extracorporeal membrane oxygenation. Pediatr. Res. 39, 209–215 (1996).
Short, B. L. I. The effect of extracorporeal life support on the brain: a focus on ECMO. Semin. Perinatol. 29, 45–50 (2005).
Billimoria, Z. C. et al. Noninvasive neurocritical care monitoring for neonates on extracorporeal membrane oxygenation: where do we stand? J. Perinatol. 41, 830–835 (2020).
Liem, K. D. et al. Cerebral oxygenation and hemodynamics during induction of extracorporeal membrane oxygenation as investigated by near infrared spectrophotometry. Pediatrics 95, 555–561 (1995).
van Heijst, A., Liem, D., Hopman, J., Van Der Staak, F. & Sengers, R. Oxygenation and hemodynamics in left and right cerebral hemispheres during induction of veno-arterial extracorporeal membrane oxygenation. J. Pediatr. 144, 223–228 (2004).
Ejike, J. C., Schenkman, K. A., Seidel, K., Ramamoorthy, C. & Roberts, J. S. Cerebral oxygenation in neonatal and pediatric patients during veno-arterial extracorporeal life support. Pediatr. Crit. Care Med. 7, 154–158 (2006).
Papademetriou, M. D., Tachtsidis, I., Elliot, M. J., Hoskote, A. & Elwell, C. E. Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. J. Biomed. Opt. 17, 067008 (2012).
Clair, M.-P. et al. Prognostic value of cerebral tissue oxygen saturation during neonatal extracorporeal membrane oxygenation. PLoS ONE 12, e0172991 (2017).
Tian, L. et al. Ischemia-induced Drp1 and Fis1-mediated mitochondrial fission and right ventricular dysfunction in pulmonary hypertension. J. Mol. Med. 95, 381–393 (2017).
Acknowledgements
Financial support of publication costs by the European Society for Paediatric Research (ESPR) is gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Contributions
All members of the European Special Interest Group “Near-InfraRed Spectroscopy” have substantially contributed to the conception and revision of the manuscript and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levy, P.T., Pellicer, A., Schwarz, C.E. et al. Near-infrared spectroscopy for perioperative assessment and neonatal interventions. Pediatr Res 96, 922–932 (2024). https://doi.org/10.1038/s41390-021-01791-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01791-1
This article is cited by
-
Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy
Pediatric Research (2024)
-
Association of sedation and anesthesia on cognitive outcomes in very premature infants: a retrospective observational study
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2023)
-
The effects of cerebral oximetry in mechanically ventilated newborns: a protocol for the SafeBoosC-IIIv randomised clinical trial
Trials (2023)