Abstract
Pulmonary artery acceleration time (PAT) and PAT: ejection time (PATET) ratio are echocardiographic measurements of pulmonary arterial hypertension (PAH). These noninvasive quantitative measurements are ideal to follow longitudinally through the clinical course of PAH, especially as it relates to the need for and/or response to treatment. This review article focuses on the current literature of PATET measurement for infants and children as it relates to the shortening of the PATET ratio in PAH. At the same time, further development of PATET as an outcome measure for PAH in preclinical models, particularly mice, such that the field can move forward to human clinical studies that are both safe and effective. Here, we present what is known about PATET in infants and children and discuss what is known in preclinical models with particular emphasis on neonatal mouse models. In both animal models and human disease, PATET allows for longitudinal measurements in the same individual, leading to more precise determinations of disease/model progression and/or response to therapy.
Impact
-
PATET ratio is a quantitative measurement by a noninvasive technique, Doppler echocardiography, providing clinicians a more precise/accurate, safe, and longitudinal assessment of pediatric PAH.
-
We present a brief history/state of the art of PATET ratio to predict PAH in adults, children, infants, and fetuses, as well as in small animal models of PAH.
-
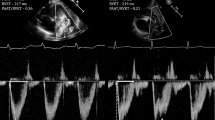
In a preliminary study, PATET shortened by 18% during acute hypoxic exposure compared to pre-hypoxia.
-
Studies are needed to establish PATET, especially in mouse models of disease, such as bronchopulmonary, as a routine measure of PAH.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Simonneau, G. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53, 1–13 (2019).
Nef, H. M., Mollmann, H., Hamm, C., Grimminger, F. & Ghofrani, H. A. Pulmonary hypertension: updated classification and management of pulmonary hypertension. Heart 96, 552–559 (2010).
Levy, P. T., Patel, M. D., Choudhry, S., Hamvas, A. & Singh, G. K. Evidence of echocardiographic markers of pulmonary vascular disease in asymptomatic infants born preterm at one year of age. J. Pediatr. 197, 48–56.e42 (2018).
Patel, M. D. et al. Echocardiographic assessment of right ventricular afterload in preterm infants: maturational patterns of pulmonary artery acceleration time over the first year of age and implications for pulmonary hypertension. J. Am. Soc. Echocardiogr. 32, 884–894.e884 (2019).
Gaulton, J. S. et al. Relationship between pulmonary artery acceleration time and pulmonary artery pressures in infants. Echocardiography 36, 1524–1531 (2019).
Koestenberger, M. et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ. Cardiovasc. Imaging 10, 1–12 (2017).
Habash, S. et al. Normal values of the pulmonary artery acceleration time (Paat) and the right ventricular ejection time (Rvet) in children and adolescents and the impact of the Paat/Rvet-index in the assessment of pulmonary hypertension. Int. J. Cardiovasc. Imaging 35, 295–306 (2019).
Gardin, J. M. et al. Effect of acute changes in heart rate on doppler pulmonary artery acceleration time in a Porcine Model. Chest 94, 994–997 (1988).
Mallery, J. A., Gardin, J. M., King, S. W., Ey, S. & Henry, W. L. Effects of heart rate and pulmonary artery pressure on doppler pulmonary artery acceleration time in experimental acute pulmonary hypertension. Chest 100, 470–473 (1991).
Levy, P. T. et al. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J. Am. Soc. Echocardiogr. 29, 1056–1065 (2016).
Wang, Y. C., Huang, C. H. & Tu, Y. K. Pulmonary hypertension and pulmonary artery acceleration time: a systematic review and meta-analysis. J. Am. Soc. Echocardiogr. 31, 201–210 e203 (2018).
Kitabatake, A. et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 68, 302–309 (1983).
Dabestani, A. et al. Evaluation of pulmonary artery pressure and resistance by pulsed doppler echocardiography. Am. J. Cardiol. 59, 662–668 (1987).
Chan, K. L. et al. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J. Am. Coll. Cardiol. 9, 549–554 (1987).
Matsuda, M. et al. Reliability of non-invasive estimates of pulmonary hypertension by pulsed Doppler echocardiography. Br. Heart J. 56, 158–164 (1986).
Marra, A. M. et al. Reference ranges and determinants of right ventricle outflow tract acceleration time in healthy adults by two-dimensional echocardiography. Int. J. Cardiovasc. Imaging 33, 219–226 (2017).
Tossavainen, E. et al. Pulmonary artery acceleration time in identifying pulmonary hypertension patients with raised pulmonary vascular resistance. Eur. Heart J. Cardiovasc. Imaging 14, 890–897 (2013).
Yared, K. et al. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J. Am. Soc. Echocardiogr. 24, 687–692 (2011).
Granstam, S. O., Bjorklund, E., Wikstrom, G. & Roos, M. W. Use of echocardiographic pulmonary acceleration time and estimated vascular resistance for the evaluation of possible pulmonary hypertension. Cardiovasc. Ultrasound 11, 7 (2013).
Tousignant, C. & Van Orman, J. R. Pulmonary artery acceleration time in cardiac surgical patients. J. Cardiothorac. Vasc. Anesth. 29, 1517–1523 (2015).
Cowie, B., Kluger, R., Rex, S. & Missant, C. The relationship between pulmonary artery acceleration time and mean pulmonary artery pressure in patients undergoing cardiac surgery: an observational study. Eur. J. Anaesthesiol. 33, 28–33 (2016).
Zhan, H. Y., Xu, F. Q., Liu, C. X. & Zhao, G. Clinical applicability of monitoring pulmonary artery blood flow acceleration time variations in monitoring fetal pulmonary artery pressure. Adv. Clin. Exp. Med. 27, 1723–1727 (2018).
Ernst, J. H. & Goldberg, S. J. Do changes in fraction of inspired oxygen affect pulmonary artery acceleration time? Am. J. Cardiol. 65, 252–254 (1990).
Moreira, H. T. et al. Pulmonary artery acceleration time in young adulthood and cardiovascular outcomes later in life: the coronary artery risk development in young adults study. J. Am. Soc. Echocardiogr. 33, 82–89.e81 (2020).
Kosturakis, D., Goldberg, S. J., Allen, H. D. & Loeber, C. Doppler echocardiographic prediction of pulmonary arterial hypertension in congenital heart disease. Am. J. Cardiol. 53, 1110–1115 (1984).
Damy, T. et al. Pulmonary acceleration time to optimize the timing of lung transplant in cystic fibrosis. Pulm. Circ. 2, 75–83 (2012).
Mourani, P. M., Sontag, M. K., Younoszai, A., Ivy, D. D. & Abman, S. H. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 121, 317–325 (2008).
Mohammad Nijres, B. et al. Utility of pulmonary artery acceleration time to estimate systolic pulmonary artery pressure in neonates and young infants. Pediatr. Cardiol. 41, 265–271 (2020).
Carlton, E. F. et al. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J. Pediatr. 186, 29–33 (2017).
Gill, A. B. & Weindling, A. M. Raised pulmonary artery pressure in very low birthweight infants requiring supplemental oxygen at 36 weeks after conception. Arch. Dis. Child Fetal Neonatal Ed. 72, F20–F22 (1995).
Gill, A. B. & Weindling, A. M. Pulmonary artery pressure changes in the very low birthweight infant developing chronic lung disease. Arch. Dis. Child 68, 303–307 (1993).
Subhedar, N. V., Hamdan, A. H., Ryan, S. W. & Shaw, N. J. Pulmonary artery pressure: early predictor of chronic lung disease in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 78, F20–F24 (1998).
Su, B. H., Watanabe, T., Shimizu, M. & Yanagisawa, M. Doppler assessment of pulmonary artery pressure in neonates at risk of chronic lung disease. Arch. Dis. Child Fetal Neonatal Ed. 77, F23–F27 (1997).
Sehgal, A., Blank, D., Roberts, C. T., Menahem, S. & Hooper, S. B. Assessing pulmonary circulation in severe bronchopulmonary dysplasia using functional echocardiography. Physiol. Rep. 9, e14690 (2021).
Machado, M. V., Chita, S. C. & Allan, L. D. Acceleration time in the aorta and pulmonary artery measured by doppler echocardiography in the midtrimester normal human fetus. Br. Heart J. 58, 15–18 (1987).
Fuke, S. et al. Antenatal prediction of pulmonary hypoplasia by acceleration time/ejection time ratio of fetal pulmonary arteries by Doppler blood flow velocimetry. Am. J. Obstet. Gynecol. 188, 228–233 (2003).
Chaoui, R. et al. Doppler echocardiography of the main stems of the pulmonary arteries in the normal human fetus. Ultrasound Obstet. Gynecol. 11, 173–179 (1998).
Rasanen, J., Huhta, J. C., Weiner, S., Wood, D. C. & Ludomirski, A. Fetal Branch pulmonary arterial vascular impedance during the second half of pregnancy. Am. J. Obstet. Gynecol. 174, 1441–1449 (1996).
Azpurua, H. et al. Acceleration/ejection time ratio in the fetal pulmonary artery predicts fetal lung maturity. Am. J. Obstet. Gynecol. 203, 40 e41–40.e48 (2010).
Reinero, C. et al. Acvim Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern Med 34, 549–573 (2020).
Visser, L. C., Im, M. K., Johnson, L. R. & Stern, J. A. Diagnostic value of right pulmonary artery distensibility index in dogs with pulmonary hypertension: comparison with doppler echocardiographic estimates of pulmonary arterial pressure. J. Vet. Intern. Med. 30, 543–552 (2016).
Serres, F. et al. Diagnostic value of echo-Doppler and tissue doppler imaging in dogs with pulmonary arterial hypertension. J. Vet. Intern. Med. 21, 1280–1289 (2007).
Schober, K. E. & Baade, H. Doppler echocardiographic prediction of pulmonary hypertension in west highland white terriers with chronic pulmonary disease. J. Vet. Intern. Med. 20, 912–920 (2006).
Ciuclan, L. et al. A novel murine model of severe pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 184, 1171–1182 (2011).
Jones, J. E. et al. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am. J. Physiol. Heart Circ. Physiol. 283, H364–H371 (2002).
Brittain, E., Penner, N. L., West, J. & Hemnes, A. Echocardiographic assessment of the right heart in mice. J. Vis. Exp. 1–8 (2013).
Kohut, A., Patel, N. & Singh, H. Comprehensive echocardiographic assessment of the right ventricle in murine models. J. Cardiovasc. Ultrasound 24, 229–238 (2016).
Moceri, P. et al. Imaging in pulmonary hypertension: focus on the role of echocardiography. Arch. Cardiovasc. Dis. 107, 261–271 (2014).
Urboniene, D., Haber, I., Fang, Y. H., Thenappan, T. & Archer, S. L. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L401–L412 (2010).
Badea, C. T., Bucholz, E., Hedlund, L. W., Rockman, H. A. & Johnson, G. A. Imaging methods for morphological and functional phenotyping of the rodent heart. Toxicol. Pathol. 34, 111–117 (2006).
Rey-Parra, G. J. et al. Blunted hypoxic pulmonary vasoconstriction in experimental neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 178, 399–406 (2008).
Zhou, Y. Q. et al. Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol. Genomics 18, 232–244 (2004).
Bossone, E., Bodini, B. D., Mazza, A. & Allegra, L. Pulmonary arterial hypertension: the key role of echocardiography. Chest 127, 1836–1843 (2005).
Abbas, A. E. et al. A simple method for noninvasive estimation of pulmonary vascular resistance. J. Am. Coll. Cardiol. 41, 1021–1027 (2003).
Hannemann, J., Zummack, J., Hillig, J. & Boger, R. Metabolism of asymmetric dimethylarginine in hypoxia: from bench to bedside. Pulm. Circ. 10, 2045894020918846 (2020).
Thibault, H. B. et al. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ. Cardiovasc. Imaging 3, 157–163 (2010).
Zhu, Z. et al. Echocardiographic assessment of right ventricular function in experimental pulmonary hypertension. Pulm. Circ. 9, 2045894019841987 (2019).
Summer, R. et al. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L432–L438 (2009).
Shah, M., Phillips, M. R., Quintana, M., Stupp, G. & McLean, S. E. Echocardiography allows for analysis of pulmonary arterial flow in mice with congenital diaphragmatic hernia. J. Surg. Res. 221, 35–42 (2018).
Reynolds, C. L., Zhang, S., Shrestha, A. K., Barrios, R. & Shivanna, B. Phenotypic Assessment of pulmonary hypertension using high-resolution echocardiography is feasible in neonatal mice with experimental bronchopulmonary dysplasia and pulmonary hypertension: a step toward preventing chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 1597–1605 (2016).
Morty, R. E. Using experimental models to identify pathogenic pathways and putative disease management targets in bronchopulmonary dysplasia. Neonatology 117, 233–239 (2020).
Wacker, C. M. et al. The pulmonary artery acceleration time determined with the mr-race-technique: comparison to pulmonary artery mean pressure in 12 patients. Magn. Reson. Imaging 12, 25–31 (1994).
Baumgartner, H. et al. Echocardiographic assessment of valve stenosis: eae/ase recommendations for clinical practice. J. Am. Soc. Echocardiogr. 22, 1–23 (2009). quiz 101-102.
Ehrmann, D. E. et al. Echocardiographic measurements of right ventricular mechanics in infants with bronchopulmonary dysplasia at 36 weeks postmenstrual age. J. Pediatr. 203, 210–217.e211 (2018).
McCrary, A. W. et al. Differences in eccentricity index and systolic-diastolic ratio in extremely low-birth-weight infants with bronchopulmonary dysplasia at risk of pulmonary hypertension. Am. J. Perinatol. 33, 57–62 (2016).
Abraham, S. & Weismann, C. G. Left ventricular end-systolic eccentricity index for assessment of pulmonary hypertension in infants. Echocardiography 33, 910–915 (2016).
Lindsey, M. L., Kassiri, Z., Virag, J. A. I., de Castro Bras, L. E. & Scherrer-Crosbie, M. Guidelines for measuring cardiac physiology in mice. Am. J. Physiol. Heart Circ. Physiol. 314, H733–H752 (2018).
Truong, U. et al. Update on noninvasive imaging of right ventricle dysfunction in pulmonary hypertension. Cardiovasc. Diagn. Ther. 10, 1604–1624 (2020).
Pinar, I. P. & Jones, H. D. Novel imaging approaches for small animal models of lung disease (2017 Grover Conference Series). Pulm. Circ. 8, 2045894018762242 (2018).
de Boode, W. P. et al. Application of neonatologist performed echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr. Res. 84, 68–77 (2018).
Saraswat, V. Effects of anaesthesia techniques and drugs on pulmonary function. Indian J. Anaesth. 59, 557–564 (2015).
Trachsel, D., Svendsen, J., Erb, T. O. & von Ungern-Sternberg, B. S. Effects of anaesthesia on paediatric lung function. Br. J. Anaesth. 117, 151–163 (2016).
Gille, J. et al. Perioperative anesthesiological management of patients with pulmonary hypertension. Anesthesiol. Res. Pract. 2012, 356982 (2012).
Yock, P. G. & Popp, R. L. Noninvasive estimation of right ventricular systolic pressure by doppler ultrasound in patients with tricuspid regurgitation. Circulation 70, 657–662 (1984).
Subhedar, N. V. & Shaw, N. J. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch. Dis. Child Fetal Neonatal Ed. 82, F243–F247 (2000).
Chow, L. C., Dittrich, H. C., Hoit, B. D., Moser, K. M. & Nicod, P. H. Doppler assessment of changes in right-sided cardiac hemodynamics after pulmonary thromboendarterectomy. Am. J. Cardiol. 61, 1092–1097 (1988).
Mourani, P. M. et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 191, 87–95 (2015).
Benatar, A., Clarke, J. & Silverman, M. Pulmonary hypertension in infants with chronic lung disease: non-invasive evaluation and short term effect of oxygen treatment. Arch. Dis. Child Fetal Neonatal Ed. 72, F14–F19 (1995).
Groh, G. K. et al. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J. Am. Soc. Echocardiogr. 27, 163–171 (2014).
Levy, P. T., El Khuffash, A., Woo, K. V. & Singh, G. K. Right ventricular-pulmonary vascular interactions: an emerging role for pulmonary artery acceleration time by echocardiography in adults and children. J. Am. Soc. Echocardiogr. 31, 962–964 (2018).
Fitzgerald, D., Evans, N., Van Asperen, P. & Henderson-Smart, D. Subclinical persisting pulmonary hypertension in chronic neonatal lung disease. Arch. Dis. Child Fetal Neonatal Ed. 70, F118–F122 (1994).
Reller, M. D., Morton, M. J., Reid, D. L. & Thornburg, K. L. Fetal lamb ventricles respond differently to filling and arterial pressures and to in utero ventilation. Pediatr. Res. 22, 621–626 (1987).
Funding
This work was supported by NIH NHLBI K08 HL129080 (PI: J.K.T.) and the Abigail Wexner Research Institute at Nationwide Children’s Hospital.
Author information
Authors and Affiliations
Contributions
Conception and design: J.K.T., R.A.M.; data acquisition: T.A.S., H.A., R.A.M., H.W.G.; data analysis: H.W.G., C.R.C.; drafting manuscript: J.K.T., R.A.M.; revising article: J.K.T., L.D.N.; approve of the final version to be published: J.K.T., H.A., L.D.N., T.A.S., C.R.C., H.W.G., and R.A.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trittmann, J.K., Almazroue, H., Nelin, L.D. et al. PATET ratio by Doppler echocardiography: noninvasive detection of pediatric pulmonary arterial hypertension. Pediatr Res 92, 631–636 (2022). https://doi.org/10.1038/s41390-021-01840-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01840-9
This article is cited by
-
Cardioprotective effects of extracellular vesicles from hypoxia-preconditioned mesenchymal stromal cells in experimental pulmonary arterial hypertension
Stem Cell Research & Therapy (2025)
-
Usefulness and importance of echocardiography in the diagnosis of pediatric pulmonary hypertension
Journal of Cardiovascular Imaging (2024)
-
Cauterization of the root of the left coronary artery as a straightforward, large and reproducible ischemic injury model in neonatal mice
Lab Animal (2024)