Abstract
Aim
Genetic variants contribute to the pathogenesis of bronchopulmonary dysplasia (BPD). The aim of this study is to evaluate the association of 45 SNPs with BPD susceptibility in a Turkish premature infant cohort.
Methods
Infants with gestational age <32 weeks were included. Patients were divided into BPD or no-BPD groups according to oxygen need at 28 days of life, and stratified according to the severity of BPD. We genotyped 45 SNPs, previously identified as BPD risk factors, in 192 infants.
Results
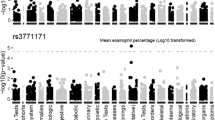
A total of eight SNPs were associated with BPD risk at allele level, two of which (rs4883955 on KLF12 and rs9953270 on CHST9) were also associated at the genotype level. Functional relationship maps suggested an interaction between five of these genes, converging on WNT5A, a member of the WNT pathway known to be implicated in BPD pathogenesis. Dysfunctional CHST9 and KLF12 variants may contribute to BPD pathogenesis through an interaction with WNT5A.
Conclusions
We suggest investigating the role of SNPs on different genes which are in relation with the Wnt pathway in BPD pathogenesis. We identified eight SNPs as risk factors for BPD in this study. In-silico functional maps show an interaction of the genes harboring these SNPs with the WNT pathway, supporting its role in BPD pathogenesis.
Trial registration
NCT03467828.
Impact
-
It is known that genetic factors may contribute to the development of BPD in preterm infants. Further studies are required to identify specific genes that play a role in the BPD pathway to evaluate them as a target for therapeutic interventions.
-
Our study shows an association of BPD predisposition with certain polymorphisms on MBL2, NFKBIA, CEP170, MAGI2, and VEGFA genes at allele level and polymorphisms on CHST9 and KLF12 genes at both allele and genotype level.
-
In-silico functional mapping shows a functional relationship of these five genes with WNT5A, suggesting that Wnt pathway disruption may play a role in BPD pathogenesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All authors accept that all data support their published claims and comply with field standards.
Material availability
All authors accept that all materials support their published claims and comply with field standards.
References
Thébaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 5, 78 (2019).
Sahni, M. & Bhandari, V. Recent advances in understanding and management of bronchopulmonary dysplasia. F1000Research 9, 703 (2020).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993 to 2012. Obstet. Anesthesia Dig. 36, 76–77 (2016).
Lapcharoensap, W. et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 169, e143676 (2015).
Thekkeveedu, R. K., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir. Med. 132, 170–177 (2017).
Bhandari, V. et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 117, 1901–1906 (2006).
Leong, M. Genetic approaches to bronchopulmonary dysplasia. NeoReviews 20, e272–e279 (2019).
Hadchouel, A. et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 184, 1164–1170 (2011).
Ryckman, K. K., Dagle, J. M., Kelsey, K., Momany, A. M. & Murray, J. C. Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonates. J. Perinatol. 32, 349–355 (2011).
Hilgendorff, A. et al. Association of polymorphisms in the mannose-binding lectin gene and pulmonary morbidity in preterm infants. Genes Immun. 8, 671–677 (2007).
Capoluongo, E. et al. Mannose-binding lectin polymorphisms and pulmonary outcome in premature neonates: a pilot study. Intens. Care Med. 33, 1787–1794 (2007).
Ali, S. et al. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J. Immunol. 190, 3949–3958 (2013).
Mailaparambil, B. et al. Genetic and epidemiological risk factors in the development of bronchopulmonary dysplasia. Dis. Markers 29, 1–9 (2010).
Kwinta, P. et al. Genetic risk factors of bronchopulmonary dysplasia. Pediatr. Res. 64, 682–688 (2008).
Wang, H. et al. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics 132, 290–297 (2013).
Torgerson, D. G. et al. Ancestry and genetic associations with bronchopulmonary dysplasia in preterm infants. Am. J. Physiol. 315, L858–L869 (2018).
Hamvas, A. et al. Exome sequencing identifies gene variants and networks associated with extreme respiratory outcomes following preterm birth. BMC Genet. 19, 94 (2018).
Li, J. et al. Exome sequencing of neonatal blood spots and the identification of genes implicated in bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 192, 589–596 (2015).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Sweet, D. G. et al. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology 115, 432–450 (2019).
Özkan, H., Erdeve, Ö. & Kutman, H. Turkish Neonatal Society guideline on the management of respiratory distress syndrome and surfactant treatment. Turk. Pediatr. Ars. 53, S45–S54 (2018).
Giusti, B. et al. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic. Res. 46, 1130–1139 (2012).
Rezvani, M. et al. Association of a FGFR-4 gene polymorphism with bronchopulmonary dysplasia and neonatal respiratory distress. Dis. Markers 35, 633–640 (2013).
Hadchouel, A. et al. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS ONE 3, e3188 (2008).
Floros, J. et al. IL-18R1 and IL-18RAP SNPs may be associated with bronchopulmonary dysplasia in African-American infants. Pediatr. Res. 71, 107–114 (2011).
Prencipe, G. et al. A polymorphism in the macrophage migration inhibitory factor promoter is associated with bronchopulmonary dysplasia. Pediatr. Res. 69, 142–147 (2011).
Sequenom. iPLEX Gold Application Guide (Matrix, Samsung, Compact) Doc. 11555 R00, CO 060190, 108 (Sequenom Inc, San Diego, CA, 2006).
Sequenom. Massarray® Assay Design 3.4 Software Guide Doc 11546, R03 CO 060094, 122 (Sequenom Inc, San Diego, CA, 2006).
Qiagen. QIAamp DNA Mini and Blood Mini HandBook 5th edn (Qiagen, 2016).
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 1989).
Barbas, C. F., Burton, D. R., Scott, J. K. & Silverman, G. J. General Procedures. Appendix 3, Phage Display (Cold Spring Harbor Laboratory Press, New York, 2001).
Ellis, J. A. & Ong, B. The Massarray® System for Targeted SNP Genotyping. Methods Mol. Biol. 1492, 77–94 (2016).
Park, C. Y. et al. Tissue-aware data integration approach for the inference of pathway interactions in metazoan organisms. Bioinformatics 31, 1093–1101 (2014).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Greene, C. S. et al. Understanding multicellular function and disease with human tissue-specific networks. Nat. Genet. 47, 569–576 (2015).
Bancalari, E. & Jain, D. Bronchopulmonary dysplasia: 50 years after the original description. Neonatology 115, 384–391 (2019).
Bonadies, L. et al. Present and future of bronchopulmonary dysplasia. J. Clin. Med. 9, 1539 (2020).
Foglia, E. E., Jensen, E. A. & Kirpalani, H. Delivery room interventions to prevent bronchopulmonary dysplasia in extremely preterm infants. J. Perinatol. Nov. 37, 1171–1179 (2017).
Principi, N., Di Pietro, G. M. & Esposito, S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 16, 36 (2018).
Ramírez-Bello, J. & Jiménez-Morales, M. Implicaciones Funcionales De Los Polimorfismos de Un Solo Nucleótido (SNP) en Genes Codificantes de Proteínas y No Codificantes en Enfermedades Multifactoriales [Functional implications of single nucleotide polymorphisms (Snps) in protein-coding and non-coding RNA genes in multifactorial diseases]. Gaceta Medica de Mexico 153, 238–250 (2017).
Lavoie, P. M. & Dubé, M. P. Genetics of bronchopulmonary dysplasia in the age of genomics. Curr. Opin. Pediatr. 22, 134–138 (2010).
Lal, C. V., Bhandari, V. & Ambalavanan, N. Genomics, microbiomics, proteomics, and metabolomics in bronchopulmonary dysplasia. Semin. Perinatol. 42, 425–431 (2018).
Lal, C. V. & Ambalavanan, N. Genetic predisposition to bronchopulmonary dysplasia. Semin. Perinatol. 39, 584–591 (2015).
Carrera, P. et al. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: a pilot study. Clin. Chim. Acta 451, 39–45 (2015).
Hiraoka, N., Misra, A., Belot, F., Hindsgaul, O. & Fukuda, M. Molecular cloning and expression of two distinct human N-acetylgalactosamine 4-O-sulfotransferases that transfer sulfate to Galnac 1 4glcnac 1 R in both N- and O-glycans. Glycobiology 11, 495–504 (2001).
Kang, H. G., Evers, M. R., Xia, G., Baenziger, J. U. & Schachner, M. Molecular cloning and expression of an N-acetylgalactosamine-4-O-sulfotransferase that transfers sulfate to terminal and non-terminal β1,4-linkedN-acetylgalactosamine. J. Biol. Chem. 276, 10861–10869 (2001).
Walter, M. J. et al. Acquired copy number alterations in adult acute myeloid leukemia genomes. Proc. Natl Acad. Sci. USA 106, 12950–12955 (2009).
Chen, J. et al. A frameshift variant in the CHST9 gene identified by family-based whole genome sequencing is associated with schizophrenia in Chinese population. Sci. Rep. 9, 12717 (2019).
Yuan, J. et al. CHST9 rs1436904 genetic variant contributes to prognosis of triple-negative breast cancer. Sci. Rep. 7, 11802 (2017).
Sweet, D. R., Fan, L., Hsieh, P. N. & Jain, M. K. Krüppel-like factors in vascular inflammation: mechanistic insights and therapeutic potential. Front. Cardiovasc. Med. 5, 6 (2018).
Godin-Heymann, N. et al. Tumour-suppression function of KLF12 through regulation of anoikis. Oncogene 35, 3324–3334 (2015).
Wan, H. et al. Kruppel-like factor 5 is required for perinatal lung morphogenesis and function. Development 135, 2563–2572 (2008).
Li, C., Xiao, J., Hormi, K., Borok, Z. & Minoo, P. Wnt5a participates in distal lung morphogenesis. Dev. Biol. 248, 68–81 (2002).
Kumawat, K. et al. TGF-β-activated kinase 1 (TAK1) signaling regulates TGF-β-induced WNT-5A expression in airway smooth muscle cells via Sp1 and β-catenin. PLoS ONE 9, e94801 (2014).
Loscertales, M., Mikels, A. J., Hu, J. K.-H., Donahoe, P. K. & Roberts, D. J. Chick pulmonary WNT5A directs airway and vascular tubulogenesis. Development 135, 1365–1376 (2008).
Li, C. et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev. Biol. 287, 86–97 (2005).
Raslan, A. A. & Yoon, J. K. WNT signaling in lung repair and regeneration. Mol. Cells 43, 774–783 (2020).
Kuypers, E. et al. Altered canonical wingless-Int signaling in the ovine fetal lung after exposure to intra-amniotic lipopolysaccharide and antenatal betamethasone. Pediatr. Res. 75, 281–287 (2014).
Frank, D. B. et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 17, 2312–2325 (2016).
Li, C., Bellusci, S., Borok, Z. & Minoo, P. Non-canonical WNT signaling in the lung. J. Biochem. 158, 355–365 (2015).
Sucre, J. et al. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am. J. Respir. Crit. care Med. 201, 1249–1262 (2020).
Ota, C., Baarsma, H. A., Wagner, D. E., Hilgendorff, A. & Königshoff, M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol. Cell. Pediatr. 3, 34 (2016).
Baarsma, H. A. et al. Correction: noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 214, 565–565 (2017).
Vuga, L. J. et al. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am. J. Respir. Cell Mol. Biol. 41, 583–589 (2009).
Li, C. et al. WNT5a-ROR signaling is essential for alveologenesis. Cells 9, 384 (2020).
Yuan, K. et al. Loss of endothelium-derived wnt5a is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation 139, 1710–1724 (2019).
Chao, C. M. et al. Targeting bronchopulmonary dysplasia-associated pulmonary hypertension (BPD-PH): potential role of the FGF signaling pathway in the development of the pulmonary vascular system. Cells 9, 1875 (2020).
Davis, C. W. et al. Expression of nitric oxide synthases and endogenous NO metabolism in bronchopulmonary dysplasia. Pediatr. Pulmonol. 43, 703–709 (2008).
Auten, R. L. et al. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am. J. Respir. Crit. Care Med. 176, 291–299 (2007).
Cetinkaya, M. et al. O-213 Association of E-nos gene polymorphism in development of bronchopulmonary dysplasia. Archiv. Dis. Childhood 99, A105–A106 (2014).
Cárdenes, N. et al. Human ex vivo lung perfusion: a novel model to study human lung diseases. Sci. Rep. 11, 490 (2021).
Matsumura, K. & Ito, S. Novel biomarker genes which distinguish between smokers and chronic obstructive pulmonary disease patients with machine learning approach. BMC Pulm. Med. 20, 29 (2020).
Lingappan, K. & Savani, R. C. The Wnt signaling pathway and the development of bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 201, 1174–1176 (2020).
Acknowledgements
This study was supported by a grant from the Scientific Research Project Programme of the Yıldız Technical University with a project number of 2016-07-04-DOP02.
Author information
Authors and Affiliations
Contributions
A.A.; conceptualization, methodology, acquisition of data, formal analysis and investigation, resources, and writing—original draft preparation, S.Y.S; conceptualization, methodology, acquisition of data, and writing—original draft preparation, O.M.U.; formal analysis and investigation, and resources, A.E.; formal analysis and investigation, and resources. M.C.; conceptualization, methodology, acquisition of data, and writing—review and editing. O.D.; writing—review, editing, and providing the final version of the manuscript. D.T.-B.; conceptualization, methodology, acquisition of data, formal analysis and investigation, resources, and writing—original draft preparation, funding, and supervision.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local Ethics Committee of Kanuni Sultan Suleyman Training and Research Hospital (KAEK/2016.12.31).
Competing interests
The authors declare no competing interests.
Consent to participate
Written and signed informed consent was taken from all parents of all included infants.
Consent for publication
All authors approve and give consent for this version to be published.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akat, A., Yilmaz Semerci, S., Ugurel, O.M. et al. Bronchopulmonary dysplasia and wnt pathway-associated single nucleotide polymorphisms. Pediatr Res 92, 888–898 (2022). https://doi.org/10.1038/s41390-021-01851-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01851-6