Abstract
Purpose
To explore the effectiveness of flexible bronchoscopy in pediatric Mycoplasma pneumoniae pneumonia (MPP).
Methods
This retrospective cohort study included children with MPP admitted between 2016 and 2019 in Shanghai. Tracheobronchial manifestations, etiologic findings, therapeutic effect, and health-economic indicators were assessed in bronchoscopy (plus bronchoalveolar lavage (BAL)) and non-bronchoscopy group. We used propensity-score matching and multivariable logistic regression to investigate the effect of bronchoscopy and BAL on disease recovery.
Results
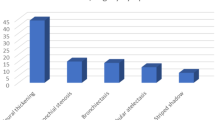
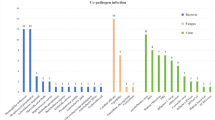
In 900 children with MPP, 24/278 (8.6%) of those who underwent bronchoscopy had sputum plugs. Coinfection rate was four-fold enhanced by BAL (19.6% vs. 4.5%, p < 0.01) in patients with severe MPP (SMPP) and nearly doubled (10.8% vs. 5.9%, p = 0.03) in those without SMPP, compared with no BAL. Total of 224 (24.9%) patients had multilobar consolidation; after BAL, a significantly shorter lesion-resolution duration was observed on imaging (OR: 0.2, 95% CI: 0.0−0.7). However, longer fever duration (OR: 2.8, 95% CI: 1.7−4.8), hospital stay (OR: 3.1, 95% CI: 1.9−5.1), and higher costs were found in the bronchoscopy group than in the non-bronchoscopy group.
Conclusions
Through BAL, coinfection may explain one-fifth of causes for SMPP. Bronchoscopy with BAL may increase the detection rate of pathogen and resolve pulmonary lesions in patients with multilobar consolidation.
Impact
-
Flexible bronchoscopy with bronchoalveolar lavage is of great assistance in the timely detection of coinfection, sputum plug and inflammatory polyps in children with Mycoplasma pneumoniae pneumonia (MPP), and improves the recovery of lung damage in MPP patients with multilobar consolidation.
-
This study provides new insights into the indications of flexible bronchoscopy for the diagnosis and treatment of pediatric patients with MPP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015).
Morozumi, M., Takahashi, T. & Ubukata, K. Macrolide-resistant mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 16, 78–86 (2010).
Tamura, A. et al. Methylprednisolone pulse therapy for refractory mycoplasma pneumoniae pneumonia in children. J. Infect. 57, 223–228 (2008).
Zhang, Y. et al. The clinical characteristics and predictors of refractory Mycoplasma Pneumoniae pneumonia in children. PLoS ONE 11, e0156465 (2016).
Kutty, P. K. et al. Mycoplasma Pneumoniae among children hospitalized with community-acquired pneumonia. Clin. Infect. Dis. 68, 5–12 (2019).
Al-Zaidy, S. A., MacGregor, D., Mahant, S., Richardson, S. E. & Bitnun, A. Neurological complications of Pcr-proven M. Pneumoniae infections in children: prodromal illness duration may reflect pathogenetic mechanism. Clin. Infect. Dis. 61, 1092–1098 (2015).
Olson, D. et al. Outbreak of Mycoplasma Pneumoniae-associated Stevens−Johnson syndrome. Pediatrics 136, e386–e394 (2015).
Yamazaki, T. & Kenri, T. Epidemiology of Mycoplasma Pneumoniae infections in Japan and therapeutic strategies for macrolide-resistant M. Pneumoniae. Front. Microbiol. 7, 693 (2016).
Waites, K. B. et al. Macrolide-resistant Mycoplasma Pneumoniae in the United States as determined from a National Surveillance Program. J. Clin. Microbiol. 57, e00968-19 (2019).
Lee, K. L. et al. Severe Mycoplasma Pneumoniae pneumonia requiring intensive care in children, 2010−2019. J. Formos. Med. Assoc. 120, 281–291 (2021).
Han, M. S. et al. Contribution of co-detected respiratory viruses and patient age to the clinical manifestations of Mycoplasma Pneumoniae pneumonia in children. Pediatr. Infect. Dis. J. 37, 531–536 (2018).
Loens, K., Van Heirstraeten, L., Malhotra-Kumar, S., Goossens, H. & Ieven, M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J. Clin. Microbiol. 47, 21–31 (2009).
Huang, L. et al. Independent predictors for longer radiographic resolution in patients with refractory Mycoplasma Pneumoniae pneumonia: a prospective cohort study. BMJ Open 8, e023719 (2018).
Miller, J. M. et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 67, e1–e94 (2018).
Xu, X. F., Li, X. J., Liu, J. L., Wu, L. & Chen, Z. M. Serum cytokine profile contributes to discriminating M. Pneumoniae pneumonia in children. Cytokine 86, 73–78 (2016).
Murdoch, D. R., O’Brien, K. L., Driscoll, A. J., Karron, R. A. & Bhat, N. Laboratory methods for determining pneumonia etiology in children. Clin. Infect. Dis. 54, S146–S152 (2012).
Li, Y. et al. Application of metagenomic next-generation sequencing for bronchoalveolar lavage diagnostics in critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 39, 369–374 (2019).
Faro, A. et al. Official American Thoracic Society Technical Standards: flexible airway endoscopy in children. Am. J. Respir. Crit. Care Med. 191, 1066–1080 (2015).
Nie, X., Cai, G. & Li, Q. Bronchoscopy in China: the Chinese Society of Respiratory Diseases Survey. Chest 136, 1186–1187 (2009).
Midulla, F. et al. Flexible endoscopy of paediatric airways. Eur. Respir. J. 22, 698–708 (2003).
Andrés-Martín, A. et al. Consensus document on community-acquired pneumonia in children. Senp-Separ-Seip. Archivos de. Bronconeumol.ía (Engl. Ed.) 56, 725–741 (2020).
National Health Commission of the People’s Republic of China, State Administration of Traditional Chinese Medcine. Guideline for diagnosis and treatment of community-acquired pneumonia in Children (2019 version). Chin. J. Clin. Infec. Dis. 12, 6–13 (2019).
Harris, M. et al. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Children: update 2011. Thorax 66(Suppl 2), ii1–ii23 (2011).
Bradley, J. S. et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76 (2011).
Xu, Q. et al. Prediction of bronchial mucus plugs formation in patients with refractory Mycoplasma Pneumoniae pneumonia. J. Trop. Pediatr. 63, 148–154 (2017).
Yan, Y., Wei, Y., Jiang, W. & Hao, C. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma Pneumoniae pneumonia in children. Sci. Rep. 6, 39929 (2016).
Wang, L. et al. The early examination of combined serum and imaging data under flexible fiberoptic bronchoscopy as a novel predictor for refractory Mycoplasma Pneumoniae pneumonia diagnosis. Medicine 96, e9364 (2017).
Prince, O. A., Krunkosky, T. M., Sheppard, E. S. & Krause, D. C. Modelling persistent Mycoplasma Pneumoniae infection of human airway epithelium. Cell Microbiol. 20, 1066–1080 (2018).
Dincer, I., Demir, A., Akin, H., Melek, H. & Altin, S. A giant endobronchial inflammatory polyp. Ann. Thorac. Surg. 80, 2353–2356 (2005).
McShane, D. et al. Inflammatory endobronchial polyps in childhood: clinical spectrum and possible link to mechanical ventilation. Pediatr. Pulmonol. 34, 79–84 (2002).
Arancibia, F. et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am. J. Respir. Crit. Care Med. 162, 154–160 (2000).
John, S. D., Ramanathan, J. & Swischuk, L. E. Spectrum of clinical and radiographic findings in pediatric Mycoplasma Pneumonia. Radiographics 21, 121–131 (2001).
Kapur, N. et al. Therapeutic bronchoscopy in a child with sand aspiration and respiratory failure from near drowning—case report and literature review. Pediatr. Pulmonol. 44, 1043–1047 (2009).
Wu, W. Z. & Liu, D. C. Observation of the efficacy of bronchoscopic alveolar lavage for severe Mycoplasmal Pneumonia in children and the changes in lung function. J. Pract. Med. 35, 132–135 (2019).
Shao, M. K., Zhou, Y., Du, K., Zhang, Y. & Deng, L. Treatment of children with severe Mycoplasma Pneumonia by electronic bronchoscope. Chin. J. Nosocomiol. 26, 4735–4737 (2016).
Mehta, A. C. & Minai, O. A. Infection control in the bronchoscopy suite. A review. Clin. Chest Med. 20, 19–32, ix (1999).
Acknowledgements
We thank all participants and staff of this study and the physicians at the Xinhua Hospital affiliated to Shanghai Jiao Tong University School of Medicine. This work was supported by the National Natural Science Foundation of China under grant numbers 81874265 and 82073561; Research Physician of the Peak Plateau Project of Shanghai Municipal Education Commission under grant number 2020002; Shanghai Science and Technology Commission under grant numbers 18411966600 and 19410740800; National Respiratory Field Key Laboratory Emergency Project numbers EKPG21-08 and National Ministry of Science and Technology-National Key R&D Program Project numbers 2021YFE0201900.
Funding
This work was supported by the National Natural Science Foundation of China under grant numbers 81874265 and 82073561; Research Physician of the Peak Plateau Project of Shanghai Municipal Education Commission under grant number 2020002; Shanghai Science and Technology Commission under grant numbers 18411966600 and 19410740800; National Respiratory Field Key Laboratory Emergency Project numbers EKPG21-08 and National Ministry of Science and Technology-National Key R&D Program Project numbers 2021YFE0201900.
Author information
Authors and Affiliations
Contributions
L.W. and Q.X. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. S.X., H.L., L.Z. and J.A. collected data, carried out the initial analyses, and reviewed and revised the manuscript. Q.L., C.C., and X.Z. coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. L.H. and W.Z. designed the study and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for the content of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics Committee of Xinhua Hospital (XHEC-C-2018-107). This study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, L., Xie, Q., Xu, S. et al. The role of flexible bronchoscopy in children with Mycoplasma pneumoniae pneumonia. Pediatr Res 93, 198–206 (2023). https://doi.org/10.1038/s41390-021-01874-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01874-z
This article is cited by
-
Clinical score for early escalation in pediatric A2063G Mycoplasma pneumoniae pneumonia: a retrospective cohort study
BMC Infectious Diseases (2025)
-
A causal forest model integrating quantitative CT scores to predict benefit from flexible bronchoscopy in pediatric Mycoplasma pneumoniae pneumonia: a two-center retrospective study
Respiratory Research (2025)
-
Mycoplasma pneumoniae pneumonia-associated thromboembolism with plastic bronchitis: a series of five case reports and literature review
Italian Journal of Pediatrics (2024)
-
Early predictors of delayed radiographic resolution of lobar pneumonia caused by Mycoplasma pneumoniae in children: a retrospective study in China
BMC Infectious Diseases (2024)
-
Predictive value of bronchoscopy combined with CT score for refractory mycoplasma pneumoniae pneumonia in children
BMC Pulmonary Medicine (2024)