Abstract
Background
Sodium bicarbonate (NaHCO3) is no longer recommended by the Neonatal Resuscitation Program (NRP), but is still being used by some neonatologists. The effects of NaHCO3 on cerebral hemodynamics are unclear. Therefore, we investigated the effects of NaHCO3 on cerebral blood flow (CBF) and cerebrovascular function using a newborn piglet model.
Methods
Newborn pigs were anesthetized, intubated, and ventilated. Cranial windows were implanted to evaluate changes in pial arteriolar diameters (PADs) as a surrogate for CBF during a 4-h intravenous infusion of 3% NaHCO3. Cerebrovascular reactivity to vasodilators and vasoconstrictors was investigated during vehicle control and during NaHCO3 infusion.
Results
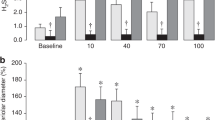
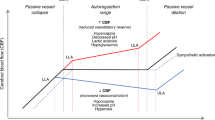
NaHCO3 infusion caused significant and progressive pial arteriolar vasoconstrictions. During NaHCO3 infusion, cerebrovascular reactivity was preserved. Adding vasodilators decreased cerebral vasoconstriction, while adding vasoconstrictors exaggerated cerebral vasoconstriction.
Conclusions
Intravenous infusion of NaHCO3 over 4 h caused progressive vasoconstriction of pial arterioles. Cerebrovascular function evaluated by the responses of pial arterioles to physiologically relevant vasoconstrictors and vasodilators was preserved during NaHCO3 infusion. A notable additional reduction of PADs was observed during NaHCO3 infusion in the presence of vasoconstrictors. Extrapolating our findings to human neonates should alarm the clinicians that using NaHCO3 in neonates may cause cerebral hypoperfusion.
Impact
-
Cerebral vasoconstriction occurs during slow infusion of 3% diluted NaHCO3.
-
Cerebral vasoconstriction is exaggerated when another vasoconstrictor is added during NaHCO3 infusion.
-
Cerebrovascular function is preserved during NaHCO3 infusion.
-
Clinicians should be aware of the risk of cerebral hypoperfusion with NaHCO3 infusion in vulnerable neonates.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bloom, R. Textbook of Neonatal Resuscitation (American Heart Association, 1987).
Gaskell, W. H. On the tonicity of the heart and blood vessels. J. Physiol. 3, 48–92 16 (1880).
Orchard, C. H. & Kentish, J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 258, C967–C981 (1990).
Howell, J. H. Sodium bicarbonate in the perinatal setting–revisited. Clin. Perinatol. 14, 807–16. (1987).
Kette, F., Weil, M. H., von Planta, M., Gazmuri, R. J. & Rackow, E. C. Buffer agents do not reverse intramyocardial acidosis during cardiac resuscitation. Circulation 81, 1660–1666 (1990).
Niermeyer, S. et al. International Guidelines for Neonatal Resuscitation: An excerpt from the Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International Consensus on Science. Contributors and Reviewers for the Neonatal Resuscitation Guidelines. Pediatrics 106, E29 (2000).
Madar, J. et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 161, 291–326 (2021).
John, K. Textbook of Neonatal Resuscitation 5th edn (American Heart Association & American Academy of Pediatrics, 2006).
Lokesh, L., Kumar, P., Murki, S. & Narang, A. A randomized controlled trial of sodium bicarbonate in neonatal resuscitation-effect on immediate outcome. Resuscitation 60, 219–223 (2004).
Aschner, J. L. & Poland, R. L. Sodium bicarbonate: basically useless therapy. Pediatrics 122, 831–835 (2008).
Bourchier, D. & Weston, P. J. Metabolic acidosis in the first 14 days of life in infants of gestation less than 26 weeks. Eur. J. Pediatr. 174, 49–54 (2015).
Saenz, P. et al. A survey of intravenous sodium bicarbonate in neonatal asphyxia among European neonatologists: gaps between scientific evidence and clinical practice. Neonatology 99, 170–176 (2011).
Massenzi, L., Aufieri, R., Donno, S., Agostino, R. & Dotta, A. Neonatal Pharmacotherapy Study Group of the Italian Society of N. Use of intravenous sodium bicarbonate in neonatal intensive care units in Italy: a nationwide survey. Ital. J. Pediatr. 47, 63 (2021).
Papile, L. A., Burstein, J., Burstein, R., Koffler, H. & Koops, B. Relationship of intravenous sodium bicarbonate infusions and cerebral intraventricular hemorrhage. J. Pediatr. 93, 834–836 (1978).
Simmons, M. A., Adcock, E. W. 3rd, Bard, H. & Battaglia, F. C. Hypernatremia and intracranial hemorrhage in neonates. N. Engl. J. Med. 291, 6–10 (1974).
van Alfen-van der Velden, A. A. et al. Effects of rapid versus slow infusion of sodium bicarbonate on cerebral hemodynamics and oxygenation in preterm infants. Biol. Neonate 90, 122–127 (2006).
Katheria, A. C. et al. Hemodynamic effects of sodium bicarbonate administration. J. Perinatol. 37, 518–20. (2017).
Singer, R. B., Deering, R. C. & Clark, J. K. The acute effects in man of a rapid intravenous infusion of hypertonic sodium bicarbonate solution. II. Changes in respiration output carbon dioxide. J. Clin. Invest. 35, 245–253 (1956).
Parfenova, H. & Leffler, C. W. Effects of hypercapnia on prostanoid and cAMP production by cerebral microvascular cell cultures. Am. J. Physiol. 270, C1503–C1510 (1996).
Nnorom, C. C. et al. Contributions of KATP and KCa channels to cerebral arteriolar dilation to hypercapnia in neonatal brain. Physiol. Rep. 2 (2014).
Pourcyrous, M., Chilakala, S., Elabiad, M. T., Parfenova, H. & Leffler, C. W. Does prolonged severe hypercapnia interfere with normal cerebrovascular function in piglets? Pediatr. Res. 84, 290–295 (2018).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 40, 1769–1777 (2020).
Dobbing, J. & Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83 (1979).
Buckley, N. M., Gootman, P. M., Yellin, E. L. & Brazeau, P. Age-related cardiovascular effects of catecholamines in anesthetized piglets. Circ. Res. 45, 282–292 (1979).
Pond, W. G. & Haupt, K. A. The Biology of the Pig (Cornell University Press, 1978).
Armstead, W. M., Mirro, R., Leffler, C. W. & Busija, D. W. Influence of endothelin on piglet cerebral microcirculation. Am. J. Physiol. 257, H707–H710 (1989).
Busija, D. W., Leffler, C. W. & Beasley, D. G. Effects of leukotrienes C4, D4, and E4 on cerebral arteries of newborn pigs. Pediatr. Res. 20, 973–976 (1986).
Laptook, A. R. The effects of sodium bicarbonate on brain blood flow and O2 delivery during hypoxemia and acidemia in the piglet. Pediatr. Res. 19, 815–819 (1985).
Kety, S. S. & Schmidt, C. F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 27, 484–492 (1948).
Rapoport, S. I., Hori, M. & Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 223, 323–331 (1972).
Arvidsson, S., Haggendal, E. & Winso, I. Influence on cerebral blood flow of infusion of sodium bicarbonate during respiratory acidosis and alkalosis in the dog. Acta Anaesthesiol. Scand. 25, 146–52. (1981).
Kontos, H. A., Raper, A. J. & Patterson, J. L. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke 8, 358–360 (1977).
Britton, S. L., Lutherer, L. O. & Davies, D. G. Effect of cerebral extracellular fluid acidity on total and regional cerebral blood flow. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 818–26 (1979).
Boedtkjer, E., Hansen, K. B., Boedtkjer, D. M. B., Aalkjaer, C. & Boron, W. F. Extracellular HCO3- is sensed by mouse cerebral arteries: regulation of tone by receptor protein tyrosine phosphatase gamma. J. Cereb. Blood Flow Metab. 36, 965–980 (2016).
Nakashima, K. et al. The effect of sodium bicarbonate on CBF and intracellular pH in man: stable Xe-CT and 31P-MRS. Acta Neurol. Scand. Suppl. 166, 96–98 (1996).
Lassen, N. A. Brain extracellular pH: the main factor controlling cerebral blood flow. Scand. J. Clin. Lab. Invest. 22, 247–251 (1968).
Lou, H. C., Lassen, N. A. & Fris-Hansen, B. Decreased cerebral blood flow after administration of sodium bicarbonate in the distressed newborn infant. Acta Neurol. Scand. 57, 239–247 (1978).
Caldwell, H. G. et al. Arterial carbon dioxide and bicarbonate rather than pH regulate cerebral blood flow in the setting of acute experimental metabolic alkalosis. J. Physiol. 599, 1439–57. (2021).
Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 81, 423–428 (2005).
Pryds, A., Tonnesen, J., Pryds, O., Knudsen, G. M. & Greisen, G. Cerebral pressure autoregulation and vasoreactivity in the newborn rat. Pediatr. Res. 57, 294–298 (2005).
Leffler, C. W., Beasley, D. G. & Busija, D. W. Cerebral ischemia alters cerebral microvascular reactivity in newborn pigs. Am. J. Physiol. 257, H266–H271 (1989).
Pourcyrous, M., Parfenova, H., Bada, H. S., Korones, S. B. & Leffler, C. W. Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and recovery in newborn pigs. Pediatr. Res. 41, 617–23. (1997).
Hoiland, R. L., Fisher, J. A. & Ainslie, P. N. Regulation of the cerebral circulation by arterial carbon dioxide. Compr. Physiol. 9, 1101–54. (2019).
Messeter, K. & Siesjo, B. K. Regulation of the CSF pH in acute and sustained respiratory acidosis. Acta Physiol. Scand. 83, 21–30 (1971).
Pannier, J. L., Demeester, G. & Leusen, I. The influence of nonrespiratory alkalosis on cerebral blood flow in cats. Stroke 5, 324–329 (1974).
Abeysekara, S. et al. Infusion of sodium bicarbonate in experimentally induced metabolic acidosis does not provoke cerebrospinal fluid (CSF) acidosis in calves. Can. J. Vet. Res. 76, 16–22 (2012).
Siesjo, B. K. Symposium on acid-base homeostasis. The regulation of cerebrospinal fluid pH. Kidney Int. 1, 360–374 (1972).
Acknowledgements
We thank Courtney Bricker-Anthony for editorial assistance and Alex Fedinec for technical assistance.
Funding information
This work was supported by the NIH R01NS101717 (to H.P.) and R01NS105655 (to H.P.).
Author information
Authors and Affiliations
Contributions
S.K.C. performed the animal experiments, carried out the initial and final analysis, made the figures, drafted the initial manuscript, and reviewed the final manuscript. H.P. coordinated experiments, supervised data collection, carried out the initial and the final analysis, and reviewed the final manuscript. M.P. conceptualized and designed the study, supervised data collection, reviewed the first draft, and revised the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chilakala, S.K., Parfenova, H. & Pourcyrous, M. The effects of sodium bicarbonate infusion on cerebrovascular function in newborn pigs. Pediatr Res 92, 729–736 (2022). https://doi.org/10.1038/s41390-021-01876-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-021-01876-x