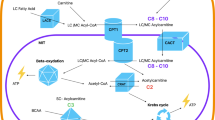
Abstract
Background
The clinical severity of very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency is difficult to predict using conventional diagnostic methods.
Methods
Peripheral blood mononuclear cells obtained from 14 VLCAD deficiency patients and 23 healthy adults were loaded with carbon-13-universally labeled (U-13C-) fatty acids. Differences in acylcarnitine ratios between the patients and healthy groups and correlations between acylcarnitine ratios and a newly established clinical severity score (CSS) in the patient group were statistically examined.
Results
There was a significant decrease in the 13C-C2/13C-C18 and 13C-C12/13C-C14 ratios in the U-13C-stearic acid loading test and in the 13C-C2/13C-C18:1 and 13C-C12:1/13C-C14:1 ratios in the U-13C-oleic acid loading test in the patient group. The values of each ratio were significantly correlated with the CSS, suggesting that they could predict disease severity. Additionally, patients with a higher 13C-C16/13C-C18 ratio than the 13C-C14/13C-C18 ratio in the U-13C-stearic acid loading test had a significantly higher CSS and were presumed to have more severe disease.
Conclusions
Our data indicated that this method could be used to predict the clinical severity of VLCAD deficiency, and identify patients at a risk of severe disease.
Impact
-
We established a novel method to predict the severity of VLCAD deficiency by performing a loading test with carbon-13-labeled fatty acids on peripheral blood mononuclear cells.
-
The U-13C-oleic acid loading test was useful for comparing the patient group with the control group in terms of disease severity.
-
The U-13C-stearic acid loading test was useful for identifying the more severely affected patients.
-
These methods are relatively less invasive and enable rapid evaluation of the clinical severity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Burrage, L. C. et al. Elevations of C14:1 and C14:2 plasma acylcarnitines in fasted children: a diagnostic dilemma. J. Pediatr. 169, 208–213 (2016).
Bo, R. et al. False positive cases of elevated tetradecenoyl carnitine in newborn mass screening showed significant loss of body weight. Mol. Genet. Metab. Rep. 24, 100634 (2020).
Shigematsu, Y. et al. Selective screening for fatty acid oxidation disorders by tandem mass spectrometry: difficulties in practical discrimination. J. Chromatogr. B 792, 63–72 (2003).
Roe, D. S., Vianey-Saban, C., Sharma, S., Zabot, M. T. & Roe, C. R. Oxidation of unsaturated fatty acids by human fibroblasts with very-long-chain acyl-CoA dehydrogenase deficiency: aspects of substrate specificity and correlation with clinical phenotype. Clin. Chim. Acta 312, 55–67 (2001).
Souri, M., Aoyama, T., Cox, G. F. & Hashimoto, T. Catalytic and FAD-binding residues of mitochondrial very long chain acyl-coenzyme A dehydrogenase. J. Biol. Chem. 273, 4227–4231 (1998).
Souri, M., Aoyama, T., Yamaguchi, S. & Hashimoto, T. Relationship between structure and substrate-chain-length specificity of mitochondrial very-long-chain acyl-coenzyme A dehydrogenase. Eur. J. Biochem. 257, 592–598 (1998).
McAndrew, R. P. et al. Structural basis for substrate fatty acyl chain specificity. J. Biol. Chem. 283, 9435–9443 (2008).
Hisahara, S. et al. A heterozygous missense mutation in adolescent-onset very long-chain acyl-CoA dehydrogenase deficiency with exercise-induced rhabdomyolysis. Tohoku J. Exp. Med. 235, 305–310 (2015).
Spiekerkoetter, U. et al. MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J. Pediatr. 143, 335–342 (2003).
Baruteau, J. et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J. Inherit. Metab. Dis. 36, 795–803 (2013).
Takusa, Y. et al. Identification and characterization of temperature-sensitive mild mutations in three japanese patients with nonsevere forms of very-long-chain acyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 75, 227–234 (2002).
Diekman, E. et al. The newborn screening paradox: sensitivity vs. overdiagnosis in VLCAD deficiency. Jimd Rep. 27, 101–106 (2016).
Pena, L. D. M. et al. Outcomes and genotype-phenotype correlations in 52 individuals with VLCAD deficiency diagnosed by NBS and enrolled in the IBEM-IS database. Mol. Genet. Metab. 118, 272–281 (2016).
Hoffmann, L., Haussmann, U., Mueller, M. & Spiekerkoetter, U. VLCAD enzyme activity determinations in newborns identified by screening: a valuable tool for risk assessment. J. Inherit. Metab. Dis. 35, 269–277 (2012).
Bouvier, D., Vianey-Saban, C., Ruet, S. & Acquaviva, C. Development of a tandem mass spectrometry method for rapid measurement of medium- and very-long-chain Acyl-CoA dehydrogenase activity in fibroblasts. Jimd Rep. 35, 71–78 (2017).
Merinero, B. et al. Four years’ experience in the diagnosis of very long-chain acyl-CoA dehydrogenase deficiency in infants detected in three Spanish Newborn Screening centers. Jimd Rep. 39, 63–74 (2018).
Zhang, R. N. et al. Clinical features and mutations in seven Chinese patients with very long chain acyl-CoA dehydrogenase deficiency. World J. Pediatr. 10, 119–125 (2014).
Pons, R. et al. Clinical and molecular heterogeneity in very-long-chain acyl-coenzyme A dehydrogenase deficiency. Pediatr. Neurol. 22, 98–105 (2000).
Andresen, B. S. et al. Cloning and characterization of human very-long-chain acyl-CoA dehydrogenase cDNA, chromosomal assignment of the gene and identification in four patients of nine different mutations within the VLCAD gene. Hum. Mol. Genet. 5, 461–472 (1996).
Yuasa, M. et al. Evaluation of metabolic defects in fatty acid oxidation using peripheral blood mononuclear cells loaded with deuterium-labeled fatty acids. Dis. Markers 2019, 2984747 (2019).
Tajima, G. et al. Development of a new enzymatic diagnosis method for very-long-chain acyl-CoA dehydrogenase deficiency by detecting 2-hexadecenoyl-CoA production and its application in tandem mass spectrometry-based selective screening and newborn screening in Japan. Pediatr. Res. 64, 667–672 (2008).
Yamada, K. et al. Open-label clinical trial of bezafibrate treatment in patients with fatty acid oxidation disorders in Japan. Mol. Genet. Metab. Rep. 15, 55–63 (2018).
Fuseya, Y. et al. Adult-onset repeat rhabdomyolysis with a very long-chain acyl-CoA dehydrogenase deficiency due to compound heterozygous ACADVL mutations. Intern. Med. 59, 2729–2732 (2020).
Shiraishi, H. et al. Efficacy of bezafibrate for preventing myopathic attacks in patients with very long-chain acyl-CoA dehydrogenase deficiency. Brain Dev. 43, 214–219 (2021).
Rydel, B. et al. The natural history of elevated tetradecenoyl-L-carnitine detected by newborn screening in New Zealand: implications for very long chain acyl-CoA dehydrogenase deficiency screening and treatment. J. Inherit. Metab. Dis. 39, 409–414 (2016).
Diekman, E. F. et al. Fatty acid oxidation flux predicts the clinical severity of VLCAD deficiency. Genet. Med. 17, 989–994 (2015).
Bleeker, J. C. et al. Impact of newborn screening for very-long-chain acyl-CoA dehydrogenase deficiency on genetic, enzymatic, and clinical outcomes. J. Inherit. Metab. Dis. 42, 414–423 (2019).
Yamada, K. et al. Serum C14:1/C12:1 ratio is a useful marker for differentiating affected patients with very long-chain acyl-CoA dehydrogenase deficiency from heterozygous carriers. Mol. Genet. Metab. Rep. 21, 100535 (2019).
Atkins, A. E., Tarini, B. A., Phillips, E. K. & Calhoun, A. R. U. L. Misclassification of VLCAD carriers due to variable confirmatory testing after a positive NBS result. J. Commun. Genet. 10, 447–451 (2019).
Spiekerkoetter, U. et al. Tandem mass spectrometry screening for very long-chain acyl-CoA dehydrogenase deficiency: the value of second-tier enzyme testing. J. Pediatr. 157, 668–673 (2010).
Hesse, J. et al. The diagnostic challenge in very-long chain acyl-CoA dehydrogenase deficiency (VLCADD). J. Inherit. Metab. Dis. 41, 1169–1178 (2018).
Spiekerkoetter, U. Mitochondrial fatty acid oxidation disorders: clinical presentation of long-chain fatty acid oxidation defects before and after newborn screening. J. Inherit. Metab. Dis. 33, 527–532 (2010).
Andresen, B. S. et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am. J. Hum. Genet. 64, 479–494 (1999).
Mathur, A. et al. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation 99, 1337–1343 (1999).
Gregersen, N. et al. Mutation analysis in mitochondrial fatty acid oxidation defects: exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship. Hum. Mutat. 18, 169–189 (2001).
Acknowledgements
We thank Ms. Komeno for her assistance in carrying out acylcarnitine analysis by MS/MS.
Funding
This study was supported in part by a Grant-in-Aid for Young Scientists No. 19K17355 from the Japan Society for the Promotion of Science (K.S.) and by the Health and Labor Sciences Research Grants for Health Research on Children, Youth and Families (G.T.).
Author information
Authors and Affiliations
Contributions
K.S. and Y.S. conceptualized and designed the study. K.S., M.Y., Y.I., and I.H. collected and analyzed the data. K.S. and Y.S. drafted the initial manuscript. Y.O. supervised the study. T.H., T.K., M.A., N.I., Y.K., F.T., R.K., and G.T. reviewed and revised the manuscript critically. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
The study was approved by The Research Ethics Committee of the University of Fukui (#20210018), and written informed consent was obtained from the parent or guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sugihara, K., Yuasa, M., Isozaki, Y. et al. Severity estimation of very-long-chain acyl-CoA dehydrogenase deficiency via 13C-fatty acid loading test. Pediatr Res 92, 1391–1399 (2022). https://doi.org/10.1038/s41390-022-01979-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-01979-z