Abstract
Background
The objective was to assess the associations of child tobacco smoke exposure (TSE) biomarkers (urinary cotinine, NNAL, and nicotelline N-oxides) and parent-reported smoking and child TSE patterns with total hospital visits, pediatric emergency department (PED) visits, urgent care (UC), revisits, and hospital admissions among 0–9-year-olds.
Methods
A convenience sample of PED/UC patients (N = 242) who presented to a large, US children’s hospital who had baseline urine samples assayed for the TSE biomarkers of interest were included. Biomarker levels were log-transformed, and linear and Poisson regression models were built.
Results
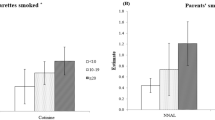
The geometric means of child cotinine, creatinine-adjusted NNAL, and N-oxide levels were 11.2 ng/ml, 30.9 pg/mg creatinine, and 24.1 pg/ml, respectively. The mean (SD) number of daily cigarettes smoked by parents was 10.2 (6.1) cigarettes. Each one-unit increase in log-NNAL levels was associated with an increase in total UC visits (aRR = 1.68, 95% CI = 1.18–2.39) among 0–9-year-olds, while controlling for the covariates. Each one-unit increase in child log-NNAL/cotinine ratio (×103) values was associated with an increase in total hospital visits (aRR = 1.39, 95% CI = 1.10–1.75) and UC visits (aRR = 1.56, 95% CI = 1.14–2.13) over 6 months.
Conclusion
Systematic screening for child TSE should be conducted during all hospital visits. The comprehensive assessment of TSE biomarkers should be considered to objectively measure young children’s exposure.
Impact
-
Higher levels of cotinine, a widely used tobacco smoke exposure biomarker, have been associated with higher healthcare utilization patterns among children.
-
Less is known on the associations of carcinogenic and tobacco smoke-derived particulate matter biomarker uptake with child healthcare utilization patterns.
-
This study assessed the associations of several biomarkers with healthcare utilization patterns among pediatric emergency department patients ages 0–9 years who lived with tobacco smokers.
-
Higher urinary NNAL biomarker levels, in individual and ratio form with cotinine, increased children’s risk for urgent care visits over 6 months.
-
Higher parent-reported cumulative child tobacco smoke exposure increased children’s risk for hospital admissions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health, Atlanta, Georgia, 2014).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum. 83, 1–1438 (2004).
World Health Organization. WHO Summary of Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals. (World Health Organization, Geneva, Switzerland, 2011).
Brody, D. J., Lu, Z. & Tsai, J. Secondhand smoke exposure among nonsmoking youth: United States, 2013-2016. NCHS Data Brief. 348, 1–8 (2019).
Merianos, A. L., Jandarov, R. A., Choi, K. & Mahabee-Gittens, E. M. Tobacco smoke exposure disparities persist in U.S. children: NHANES 1999–2014. Prev. Med. 123, 138–142 (2019).
Merianos, A. L., Jandarov, R. A. & Mahabee-Gittens, E. M. High cotinine and healthcare utilization disparities among low-income children. Am. J. Prev. Med. 60, 267–275 (2021).
Gatzke-Kopp, L. M. et al. Magnitude and chronicity of environmental smoke exposure across infancy and early childhood in a sample of low-income children. Nicotine Tob. Res. 21, 1665–1672 (2019).
Merianos, A. L., Jandarov, R. A. & Mahabee-Gittens, E. M. Secondhand smoke exposure and pediatric healthcare visits and hospitalizations. Am. J. Prev. Med. 53, 441–448 (2017).
Benowitz, N. et al. Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiol. Biomark. Prev. 19, 2795–2800 (2010).
Wei, B., Blount, B. C., Xia, B. & Wang, L. Assessing exposure to tobacco-specific carcinogen NNK using its urinary metabolite NNAL measured in US population: 2011-2012. J. Expo. Sci. Environ. Epidemiol. 26, 249–256 (2016).
Park, M. B. Living with parents who smoke predicts levels of toxicant exposure in children. Sci. Rep. 10, 11173 (2020).
Jeong, S. H., Jang, B. N., Kang, S. H., Joo, J. H. & Park, E. C. Association between parents’ smoking status and tobacco exposure in school-age children: assessment using major urine biomarkers. Sci. Rep. 11, 4536 (2021).
Jacob, P. et al. Thirdhand smoke: new evidence, challenges, and future directions. Chem. Res. Toxicol. 30, 270–294 (2017).
Chao, M. R. et al. Children are particularly vulnerable to environmental tobacco smoke exposure: evidence from biomarkers of tobacco-specific nitrosamines, and oxidative stress. Environ. Int. 120, 238–245 (2018).
Hecht, S. S. et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol. Biomark. Prev. 15, 988–992 (2006).
Stepanov, I., Hecht, S. S., Duca, G. & Mardari, I. Uptake of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by Moldovan children. Cancer Epidemiol. Biomark. Prev. 15, 7–11 (2006).
Hecht, S. S. et al. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol. Biomark. Prev. 10, 1109–1116 (2001).
Jacob, P., Goniewicz, M. L., Havel, C. M., Schick, S. F. & Benowitz, N. L. Nicotelline: a proposed biomarker and environmental tracer for particulate matter derived from tobacco smoke. Chem. Res. Toxicol. 26, 1615–1631 (2013).
Mahabee-Gittens, E. M. et al. Healthy families: study protocol for a randomized controlled trial of a screening, brief intervention, and referral to treatment intervention for caregivers to reduce secondhand smoke exposure among pediatric emergency patients. BMC Public Health 17, 374 (2017).
U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office on Smoking and Health, Atlanta, GA, 2006).
Mahabee-Gittens, E. M. et al. Contribution of thirdhand smoke to overall tobacco smoke exposure in pediatric patients: study protocol. BMC Public Health 19, 491 (2019).
Jacob, P. et al. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal. Chem. 80, 8115–8121 (2008).
Hao, S. et al. Risk prediction of emergency department revisit 30 days post discharge: a prospective study. PLoS ONE 9, e112944 (2014).
Bardach, N. S. et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics 132, 429–436 (2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2013).
Merianos, A. L., Dixon, C. A. & Mahabee-Gittens, E. M. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. J. Asthma 54, 798–806 (2017).
Benowitz, N. L. et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res 22, 1086–1097 (2020).
Goniewicz, M. L. et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol. Biomark. Prev. 18, 3421–3425 (2009).
Bernert, J. T. et al. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol. Biomark. Prev. 19, 2969–2977 (2010).
Sleiman, M. et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl Acad. Sci. USA 107, 6576–6581 (2010).
Vanker, A., Gie, R. P. & Zar, H. J. The association between environmental tobacco smoke exposure and childhood respiratory disease: a review. Expert Rev. Respir. Med. 11, 661–673 (2017).
Mahabee-Gittens, E. M. et al. Electronic health record classification of tobacco smoke exposure and cotinine levels in hospitalized pediatric patients. Hosp. Pediatr. 9, 659–664 (2019).
Brown-Johnson, C. G. & Popova, L. Exploring smoking stigma, alternative tobacco product use, & quit attempts. Health Behav. Policy Rev. 3, 13–20 (2016).
Dempsey, D. A. et al. Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Arch. Pediatr. Adolesc. Med. 166, 851–856 (2012).
Merianos, A. L., Dixon, C. A. & Mahabee-Gittens, E. M. Tobacco smoke exposure-related illnesses among pediatric emergency department patients. J. Pediatr. Health Care 31, 161–166 (2016).
Dempsey, D. A. et al. CYP2A6 genotype but not age determines cotinine half-life in infants and children. Clin. Pharmacol. Ther. 94, 400–406 (2013).
Hecht, S. S. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob. Control 13, i48–i56 (2004).
Mahabee-Gittens, E. M. et al. Utilization of a clinical decision support tool to reduce child tobacco smoke exposure in the urgent care setting. Pediatr. Emerg. Care 36, 527–531 (2020).
Kruse, G. R. & Rigotti, N. A. Routine screening of hospital patients for secondhand tobacco smoke exposure: a feasibility study. Prev. Med. 69, 141–145 (2014).
Author contributions
All authors contributed to all three of the following criteria: (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
Funding
Funded by the National Institute on Drug Abuse (NIH Grant Number K01DA044313), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH Grant Number R01HD083354), and National Institute of Environmental Health Sciences (NIH Grant Numbers R01ES027815, R01ES030743, and R21ES032161). Instrumentation and other analytical chemistry laboratory resources for the urine analyses at UCSF were supported by the National Institutes of Health (P30DA012393 and S10RR026437).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
We obtained consent from parents for their child’s participation since our sample was 0-9-years of age.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Merianos, A.L., Jandarov, R.A. & Mahabee-Gittens, E.M. Carcinogenic and tobacco smoke-derived particulate matter biomarker uptake and associated healthcare patterns among children. Pediatr Res 93, 143–153 (2023). https://doi.org/10.1038/s41390-022-02031-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02031-w