Abstract
Background
The underlying mechanisms for infantile bronchopneumonia development remain unknown.
Methods
Peripheral blood mononuclear cell (PBMCs) and serum derived from severe and mild infantile bronchopneumonia were obtained, and the expression of various molecules was detected with enzyme-linked immunosorbent assay and quantitative PCR. Such molecules were also detected in granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced bone marrow-derived NFκB2−/− dendritic cells (DCs) or NIK SMI1 (NF-κB-inducing kinase inhibitor) administrated DCs.
Results
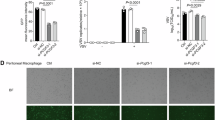
The relative mRNA expression levels of type I interferons (IFNs) (IFN-α4, IFN-β), Th17 cell-associated markers (interleukin-17A, retinoic-acid-receptor-related orphan nuclear receptor gamma, and GM-CSF), and non-canonical NF-κB member (NFκB2) were significantly up-regulated in PBMCs and DCs derived from infantile bronchopneumonia compared with healthy controls. However, compared with Th17 cell-associated markers and non-canonical NF-κB molecules, the expression of IFN-α4 and IFN-β was significantly inhibited in severe infantile bronchopneumonia compared with mild infantile bronchopneumonia. The relative protein expression of the above molecules also showed a similar expression pattern in the PBMCs or serum. NF-κB2 knockout or NIK SMI1 administration could reverse the diminished expression of IFN-β in GM-CSF-induced bone marrow-derived DCs.
Conclusions
GM-CSF-dependent non-canonical NF-κB pathway-mediated inhibition of type I IFNs production in DCs contributes to the development of severe bronchopneumonia in infant.
Impact
-
Granulocyte-macrophage colony-stimulating factor-dependent non-canonical NF-κB pathway-mediated inhibition of type I IFNs production in dendritic cells is critical for the development of infantile bronchopneumonia.
-
Our findings reveal a possible mechanism underlying the development of severe infantile bronchopneumonia.
-
The results could provide therapeutic molecular target for the treatment of such disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data could be obtained upon reasonable request to the corresponding author.
References
Reynolds, J. H. & Banerjee, A. K. Imaging pneumonia in immunocompetent and immunocompromised individuals. Curr. Opin. Pulm. Med. 18, 194–201 (2012).
de Benedictis, F. M. et al. Complicated pneumonia in children. Lancet 396, 786–798 (2020).
Li, S., Cui, W., Song, Q., Zhou, Y. & Li, J. miRNA-302e attenuates inflammation in infantile pneumonia though the RelA/BRD4/NF-κB signaling pathway. Int. J. Mol. Med. 44, 47–56 (2019).
Duan, X. et al. Comparative efficacy of Chinese herbal injections for treating pediatric bronchopneumonia: a Bayesian network meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2020, 6127197 (2020).
Cars, O. et al. Resetting the agenda for antibiotic resistance through a health systems perspective. Lancet Glob. Health 9, e1022–e1027 (2021).
Barrat, F. J., Crow, M. K. & Ivashkiv, L. B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20, 1574–1583 (2019).
Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III interferons. Immunity 50, 907–923 (2019).
Van Eyndhoven, L. C., Singh, A. & Tel, J. Decoding the dynamics of multilayered stochastic antiviral IFN-I responses. Trends Immunol. https://doi.org/10.1016/j.it.2021.07.004 (2021).
Khanna, N. R. & Gerriets, V. 2021 Interferon (StatPearls, 2021).
De Ceuninck, F., Duguet, F., Aussy, A., Laigle, L. & Moingeon, P. IFN-α: a key therapeutic target for multiple autoimmune rheumatic diseases. Drug Discov. Today 26, 2465–2473 (2021).
Guo, H., He, Z., Li, M., Wang, T. & Zhang, L. Imbalance of peripheral blood Th17 and Treg responses in children with refractory Mycoplasma pneumoniae pneumonia. J. Infect. Chemother. 22, 162–166 (2016).
Liu, M. et al. Emerging biological functions of IL-17A: a new target in chronic obstructive pulmonary disease? Front. Pharmacol. 12, 695957 (2021).
Éliás, S., Schmidt, A., Gomez-Cabrero, D. & Tegnér, J. Gene regulatory network of human GM-CSF-secreting T helper cells. J. Immunol. Res. 2021, 8880585 (2021).
El-Behi, M. et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
McGeachy, M. J. GM-CSF: the secret weapon in the TH17 arsenal. Nat. Immunol. 12, 521–522 (2011).
Tailor, P. et al. The feedback phase of type I interferon induction in dendritic cells requires interferon regulatory factor 8. Immunity 27, 228–239 (2007).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014).
Meissner, N., Rutkowski, M., Harmsen, A. L., Han, S. & Harmsen, A. G. Type I interferon signaling and B cells maintain hemopoiesis during pneumocystis infection of the lung. J. Immunol. 178, 6604–6615 (2007).
Saag, M. S. HIV infection - screening, diagnosis, and treatment. N. Engl. J. Med. 384, 2131–2143 (2021).
LeMessurier, K. S., Häcker, H., Chi, L., Tuomanen, E. & Redecke, V. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathog. 9, e1003727 (2013).
Clementi, N. et al. Viral respiratory pathogens and lung injury. Clin. Microbiol. Rev. 34, e00103-20 (2021).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 (2006).
Yoneyama, M. et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 (2005).
McGlasson, S., Jury, A., Jackson, A. & Hunt, D. Type I interferon dysregulation and neurological disease. Nat. Rev. Neurol. 11, 515–523 (2015).
Langley, J. M. & Bradley, J. S. Defining pneumonia in critically ill infants and children. Pediatr. Crit. Care Med. 6, S9–S13 (2005).
Agweyu, A., Lilford, R. J. & English, M. Appropriateness of clinical severity classification of new WHO childhood pneumonia guidance: a multi-hospital, retrospective, cohort study. Lancet Glob. Health 6, e74–e83 (2018).
Author information
Authors and Affiliations
Contributions
L.X. designed and supervised the study. Z.L. and H.W. performed experiments and analyzed data. Z.L. wrote and revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Protocol of this research was approved by the Ethics Committee of Daqing Oilfield General Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Z., Wei, H. & Li, X. Granulocyte-macrophage colony-stimulating factor suppresses induction of type I interferon in infants with severe pneumonia. Pediatr Res 93, 72–77 (2023). https://doi.org/10.1038/s41390-022-02059-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02059-y