Abstract
Background
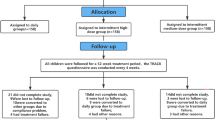
The GINA recommends inhaled corticosteroids (ICSs) for the treatment of steps 2–3 of childhood asthma. However, the difference in efficacy between these drugs remains unclear. The purpose of this study was to compare the efficacy of different ICS drugs in the treatment of childhood asthma.
Methods
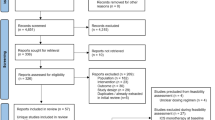
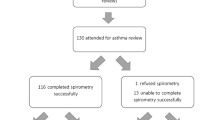
We searched PubMed and EMBASE for randomized controlled trials of ICSs in the treatment of childhood asthma. Using forced expiratory volume in the first second (FEV1) as the primary outcome, a time-course model of ICSs was constructed. In addition, the symptom-free days% were analyzed as a secondary outcome.
Results
Six studies involving 2237 children that reported FEV1 were included. The results showed that the ET50 of ciclesonide (CIC) and budesonide (BUD) was 1.23 and 2.97 weeks, respectively. Compared with them, FP had a higher efficacy. In terms of symptom-free days%, we found that the efficacy of beclometasone dipropionate was lower than that of CIC and fluticasone propionate.
Conclusion
In this study, the efficacy of three ICS drugs was quantitatively compared, providing necessary information for the implementation of medication guidelines for steps 2–3 of asthma in children.
Impact
-
This study analyzed the entire time-course of the drug efficacy of Inhaled corticosteroids in the treatment of asthma in children aged 5–12, which found that although the maximum efficacy of both ciclesonide and budesonide was the same, the onset speed of ciclesonide was faster than that of budesonide.
-
The above information provides the necessary quantitative information for the implementation of medication guidelines for steps 2–3 asthma in children.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets analyzed during this study are available in PubMed and EMBASE.
References
Global Initiative for Asthma. Global strategy for asthma management and prevention. http://www.ginasthma.org (2020).
Kramer, S., Rottier, B. L., Scholten, R. J. & Boluyt N. Ciclesonide versus other inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst. Rev. CD010352 (2013).
Mandema, J. W., Gibbs, M., Boyd, R. A., Wada, D. R. & Pfister, M. Model-based meta-analysis for comparative efficacy and safety: application in drug development and beyond. Clin. Pharm. Ther. 90, 766–769 (2011).
Mould, D. R. Model-based meta-analysis: an important tool for making quantitative decisions during drug development. Clin. Pharm. Ther. 92, 283–286 (2012).
Ito, S. Drugs for children. Clin. Pharm. Ther. 101, 704–706 (2017).
Addendum to ICH E11: clinical investigation of medicinal products in the paediatric population E11(R1). https://database.ich.org/sites/default/files/E11_R1_Addendum.pdf. Accessed September 2020.
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Leroyer, C. et al. Comparison of serial monitoring of peak expiratory flow and FEV1 in the diagnosis of occupational asthma. Am. J. Respir. Crit. Care Med. 158, 827–832 (1998).
Meibohm, B. & Derendorf, H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J. Clin. Pharm. Ther. 35, 401–413 (1997).
Holford, N. H. & Sheiner, L. B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin. Pharmacokinet. 6, 429–453 (1981).
Goutelle, S. et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharm. 22, 633–648 (2008).
Ette, E. I. & Ludden, T. M. Population pharmacokinetic modeling: the importance of informative graphics. Pharm. Res. 12, 1845–1855 (1995).
Holford, N. H., Chan, P. L., Nutt, J. G., Kieburtz, K. & Shoulson, I. Disease progression and pharmacodynamics in Parkinson disease – evidence for functional protection with levodopa and other treatments. J. Pharmacokinet. Pharmacodyn. 33, 281–311 (2006).
Kuti, J. L., Dandekar, P. K., Nightingale, C. H. & Nicolau, D. P. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharm. 43, 1116–1123 (2003).
Ette, E. I., Williams, P. J., Kim, Y. H., Lane, J. R., Liu, M.-J., & Capparelli, E. V. Model appropriateness and population pharmacokinetic modeling. J. Clin. Pharmacol. 43, 610–623 (2003).
Ette, E. I. Stability and performance of a population pharmacokinetic model. J. Clin. Pharm. 37, 486–495 (1997).
Goulooze, S. C., Galettis, P., Boddy, A. V. & Martin, J. H. Monte Carlo simulations of the clinical benefits from therapeutic drug monitoring of sunitinib in patients with gastrointestinal stromal tumours. Cancer Chemother. Pharm. 78, 209–216 (2016).
Post, T. M., Freijer, J. I., Ploeger, B. A. & Danhof, M. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharmacokinet. Pharmacodyn. 35, 185–202 (2008).
Pearlman, D. S. et al. Efficacy and safety of budesonide/formoterol pMDI vs budesonide pMDI in asthmatic children (6-< 12 years). Ann. Allergy Asthma Immunol. 118, 489–499 (2017).
Berger, W. E. et al. Effects of treatment with mometasone furoate dry powder inhaler in children with persistent asthma. Ann. Allergy Asthma Immunol. 97, 672–680 (2006).
von Berg, A. et al. Comparison of the efficacy and safety of ciclesonide 160 microg once daily vs. budesonide 400 microg once daily in children with asthma. Pediatr. Allergy Immunol. 18, 391–400 (2007).
Oliver, A. J. et al. Randomized trial of once-daily fluticasone furoate in children with inadequately controlled asthma. J. Pediatr. 178, 246–253.e242 (2016).
Pedersen, S. et al. Efficacy and safety of ciclesonide once daily and fluticasone propionate twice daily in children with asthma. Pulm. Pharm. Ther. 22, 214–220 (2009).
Escribano, A., Tutuncu, A., Löhr, I., Carlholm, M. & Polanowski, T. Clinical comparability between the CFC and HFA budesonide pressurised metered-dose inhalers in paediatric patients with asthma: a randomised controlled trial. Curr. Med. Res. Opin. 22, 1085–1092 (2006).
Arets, H. G. et al. Children with mild asthma: do they benefit from inhaled corticosteroids? Eur. Respir. J. 20, 1470–1475 (2002).
Nayak, A., Lanier, R., Weinstein, S., Stampone, P. & Welch, M. Efficacy and safety of beclomethasone dipropionate extrafine aerosol in childhood asthma: a 12-week, randomized, double-blind, placebo-controlled study. Chest 122, 1956–1965 (2002).
Płoszczuk, A., Bosheva, M., Spooner, K., McIver, T. & Dissanayake, S. Efficacy and safety of fluticasone propionate/formoterol fumarate in pediatric asthma patients: a randomized controlled trial. Ther. Adv. Respir. Dis. 12, 1753466618777924 (2018).
Korenblat, P. E. Ciclesonide and the treatment of asthma. Expert Opin. Pharmacother. 11, 463–479 (2010).
Humbert, M. Ciclesonide: a novel inhaled corticosteroid. Expert Opin. Investig. Drugs 13, 1349–1360 (2004).
Skoner, D. P. Inhaled corticosteroids: effects on growth and bone health. Ann. Allergy Asthma Immunol. 117, 595–600 (2016).
Holliday, S. M., Faulds, D. & Sorkin, E. M. Inhaled fluticasone propionate. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma. Drugs 47, 318–331 (1994).
Devonshire, A. L. & Kumar, R. Pediatric asthma: principles and treatment. Allergy Asthma Proc. 40, 389–392 (2019).
Daley-Yates, P. T. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br. J. Clin. Pharm. 80, 372–380 (2015).
Gillette, C., Rockich-Winston, N., Kuhn, J. A., Flesher, S. & Shepherd, M. Inhaler technique in children with asthma: a systematic review. Acad. Pediatr. 16, 605–615 (2016).
Acknowledgements
The authors wish to thank all the staff of the Center for Drug Clinical Research, Shanghai University of Traditional Chinese Medicine.
Funding
This was not an industry-supported study. This work received financial support from the project Shanghai S&T Innovation Plan (17401970900).
Author information
Authors and Affiliations
Contributions
H.Z.: data curation-lead, formal analysis-lead, investigation-lead, resources-lead, writing-original draft-lead. H.L.: conceptualization-supporting, formal analysis-supporting, funding acquisition-equal, methodology-supporting, writing-review and editing-supporting. Z.S.: data curation-supporting. J.Y.: data curation-supporting. Q.Z.: conceptualization-lead, funding acquisition-lead, methodology-lead, writing-review and editing-equal. L.L.: conceptualization-lead, formal analysis-supporting, funding acquisition-supporting, investigation-equal, methodology-lead, project administration-equal, software-equal, supervision-equal, validation-equal, visualization-equal, writing-original draft-supporting, writing-review and editing-lead.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, H., Liu, H., Sui, Z. et al. Quantitative comparison of different inhaled corticosteroids in the treatment of asthma in children. Pediatr Res 93, 31–38 (2023). https://doi.org/10.1038/s41390-022-02095-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02095-8