Abstract
Background
The infectious burden in hereditary spherocytosis (HS) children before splenectomy has rarely been reported and the risk of severe postsplenectomy infection is controversial.
Methods
We conducted a retrospective study of pediatric patients with HS to evaluate the risk of infection presplenectomy and postsplenectomy. The primary outcome was any bacterial, Mycoplasma, or fungal infection that required hospitalization. The secondary outcomes were sepsis and septic shock. Appendectomized children were matched on age at surgery and enrolled as controls.
Results
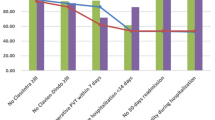
In all, 232 patients were included. Before splenectomy, the primary outcome was identified in 51 (22.0%) patients, and the secondary outcome was identified in 1 (0.4%) patient. After splenectomy, the primary and secondary outcomes were detected in 8 (4.1%) and 1 (0.5%) patients, respectively. The risk of infection was higher presplenectomy than postsplenectomy (OR, 6.6; 95% CI, 3.0–14.2). HS patients had a higher risk of infection than the controls before surgery (OR, 3.7; 95% CI, 2.3–5.9) but not after surgery (OR, 1.4; 95% CI, 0.6–3.6).
Conclusions
HS patients who require splenectomy later in life had a high incidence of hospitalization for infections. In contrast, postsplenectomy risk of hospitalization involving infection or severe infection was low.
Impact
-
Patients with hereditary spherocytosis who require splenectomy later in life have a high risk of hospital admission for infections, especially those with severe hereditary spherocytosis. With vaccines or postoperative antibiotics, splenectomy does not increase the risk of infection or severe infections.
-
Splenectomy may reduce the risk of hospitalization for infections by alleviating the complications of hereditary spherocytosis.
-
With vaccines, prophylaxis, or advanced antibiotics, the benefits of splenectomy in children with hereditary spherocytosis and a heavy disease burden may outweigh the risks.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Perrotta, S., Gallagher, P. G. & Mohandas, N. Hereditary spherocytosis. Lancet 372, 1411–1426 (2008).
Tse, W. T. & Lux, S. E. Red blood cell membrane disorders. Br. J. Haematol. 104, 2–13 (1999).
Yawata, Y. et al. Characteristic features of the genotype and phenotype of hereditary spherocytosis in the Japanese population. Int J. Hematol. 71, 118–135 (2000).
Delaunay, J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 21, 1–20 (2007).
Mohandas, N. & Chasis, J. A. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 30, 171–192 (1993).
Baird, R. N., Macpherson, A. I. & Richmond, J. Red-blood-cell survival after splenectomy in congenital spherocytosis. Lancet 2, 1060–1061 (1971).
Iolascon, A. et al. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica 102, 1304–1313 (2017).
KING, H. & SHUMACKER, H. J. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann. Surg. 136, 239–242 (1952).
Eraklis, A. J., Kevy, S. V., Diamond, L. K. & Gross, R. E. Hazard of overwhelming infection after splenectomy in childhood. N. Engl. J. Med. 276, 1225–1229 (1967).
Bisharat, N., Omari, H., Lavi, I. & Raz, R. Risk of infection and death among post-splenectomy patients. J. Infect. 43, 182–186 (2001).
Cullingford, G. L., Watkins, D. N., Watts, A. D. & Mallon, D. F. Severe late postsplenectomy infection. Br. J. Surg. 78, 716–721 (1991).
Thomsen, R. W. et al. Risk for hospital contact with infection in patients with splenectomy: a population-based cohort study. Ann. Intern. Med. 151, 546–555 (2009).
Yacobovich, J. et al. Splenectomy in childhood for non-malignant haematologic disorders - long-term follow-up shows minimal adverse effects. Br. J. Haematol. 190, 909–915 (2020).
Collaborators, G. U. M. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet 398, 870–905 (2021).
Walker, C. et al. Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416 (2013).
Bolton-Maggs, P. H., Langer, J. C., Iolascon, A., Tittensor, P. & King, M. J. Guidelines for the diagnosis and management of hereditary spherocytosis–2011 update. Br. J. Haematol. 156, 37–49 (2012).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Harris, A. M. et al. Air pollution and anemia as risk factors for pneumonia in Ecuadorian children: a retrospective cohort analysis. Environ. Health 10, 93 (2011).
Kao, J. et al. Early childhood anemia in a birth cohort in coastal Kenya: links to infection and nutrition. Am. J. Trop. Med. Hyg. 101, 242–252 (2019).
Addiss, D. G., Shaffer, N., Fowler, B. S. & Tauxe, R. V. The epidemiology of appendicitis and appendectomy in the United States. Am. J. Epidemiol. 132, 910–925 (1990).
Dursun, I., Kiziltan, M. Y., Bozkaya, D., Aygun, A. & Gucuyener, K. Pneumococcal pneumonia preceding appendicitis in a child. Eur. J. Pediatr. 163, 500 (2004).
Pedersen, F. K. Postsplenectomy infections in Danish children splenectomized 1969-1978. Acta Paediatr. Scand. 72, 589–595 (1983).
Luoto, T. T., Pakarinen, M. P. & Koivusalo, A. Long-term outcomes after pediatric splenectomy. Surgery 159, 1583–1590 (2016).
Jain, S. et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 372, 835–845 (2015).
Levy, A. et al. Anemia as a risk factor for infectious diseases in infants and toddlers: results from a prospective study. Eur. J. Epidemiol. 20, 277–284 (2005).
Martins, R. et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat. Immunol. 17, 1361–1372 (2016).
Ibrahim, M. K., Zambruni, M., Melby, C. L. & Melby, P. C. Impact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 30, 919–971 (2017).
Bhutta, Z. A. et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382, 452–477 (2013).
Rubin, L. G. & Schaffner, W. Clinical practice. Care of the asplenic patient. N. Engl. J. Med. 371, 349–356 (2014).
Kyaw, M. H. et al. Evaluation of severe infection and survival after splenectomy. Am. J. Med. 119, 271–276 (2006).
Yu, Q. et al. The impact of childhood pneumococcal conjugate vaccine immunisation on all-cause pneumonia admissions in Hong Kong: a 14-year population-based interrupted time series analysis. Vaccine 39, 2628–2635 (2021).
Dhiman, N. et al. Increased complications after appendectomy in patients with cerebral palsy: are special needs patients at risk for disparities in outcomes? Surgery 154, 479–485 (2013).
Holdsworth, R. J., Irving, A. D. & Cuschieri, A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br. J. Surg. 78, 1031–1038 (1991).
Jugenburg, M., Haddock, G., Freedman, M. H., Ford-Jones, L. & Ein, S. H. The morbidity and mortality of pediatric splenectomy: does prophylaxis make a difference? J. Pediatr. Surg. 34, 1064–1067 (1999).
Ein, S. H. et al. The morbidity and mortality of splenectomy in childhood. Ann. Surg. 185, 307–310 (1977).
Acknowledgements
We thank the patients, nurses, and administrators for their participation in the study. The authors would also like to acknowledge all doctors in our department for their contributions in the treatment of patients with hereditary spherocytosis.
Funding
This research was supported by the Natural Science Foundation of Shandong Province (grant No. ZR2020GSF118021). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y. Liu designed the study, performed the research, analyzed the data, and wrote the paper. S.J. performed the research and edited the paper. R.X., C.D., and W.P. contributed to data interpretation and reviewed and provided their comments on this manuscript. Y. Li and Y.C. designed the study, edited the paper, and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was waived by the institutional review boards due to the retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Jin, S., Xu, R. et al. Hereditary spherocytosis before and after splenectomy and risk of hospitalization for infection. Pediatr Res 93, 1336–1341 (2023). https://doi.org/10.1038/s41390-022-02229-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02229-y