Abstract
Background
In utero transmission of SARS coronavirus 2 (SARS-CoV-2) has not been fully investigated. We investigated whether newborns of mothers with COVID-19 during pregnancy might harbor SARS-CoV-2 in the gastrointestinal tract.
Methods
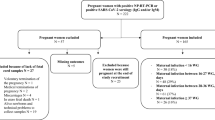
This cohort study investigated stool from 14 newborns born at 25–41 weeks admitted at delivery to our urban academic hospital whose mothers had COVID-19 during pregnancy. Eleven mothers had COVID-19 resolved more than 10 weeks before delivery. Newborn stool was evaluated for SARS-CoV-2 RNA, Spike protein, and induction of inflammatory cytokines interleukin-6 (IL-6) and interferon-γ (IFN-γ) in macrophages.
Results
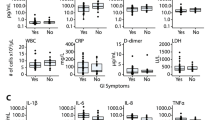
Despite negative SARS CoV-2 nasal PCRs from all newborns, viral RNAs and Spike protein were detected in the stool of 11 out of 14 newborns as early as the first day of life and increased over time in 6. Stool homogenates from all 14 newborns elicited elevated inflammatory IL-6 and IFN-γ from macrophages. Most newborns were clinically well except for one death from gestational autoimmune liver disease and another who developed necrotizing enterocolitis.
Conclusions
These findings suggest in utero transmission of SARS-CoV-2 and possible persistent intestinal viral reservoirs in the newborns. Further investigation is required to understand the mechanisms and their clinical implications.
Impact
-
SARS-CoV-2 RNAs or Spike protein was detected in the stool of 11 out of 14 preterm newborns born to mothers with resolved COVID-19 weeks prior to delivery despite negative newborn nasal PCR swabs.
-
These novel findings suggest risk of in utero SARS-CoV-2 transmission to the fetal intestine during gestation.
-
The presence of SARS-CoV-2 RNAs and Spike protein in the intestines of newborns may potentially impact the development of the gut microbiome and the immune system; the long-term health impact on the preterm infants should be further investigated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data generated or analyzed during this study are included in this article and can be requested from the corresponding author upon request.
References
Villar, J. et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 175, 817–826 (2021).
Allotey, J. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370, m3320 (2020).
Perlman, J., Oxford, C., Chang, C., Salvatore, C. & Di Pace, J. Delivery room preparedness and early neonatal outcomes during COVID-19 pandemic in New York City. Pediatrics https://doi.org/10.1542/peds.2020-1567 (2020).
Salvatore, C. M. et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc. Health 4, 721–727 (2020).
Zeng, L. et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 174, 722–725 (2020).
Cook, J. et al. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc. Health 4, 548–551 (2020).
CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 422–426 (2020).
Velazquez-Salinas, L., Verdugo-Rodriguez, A., Rodriguez, L. L. & Borca, M. V. The role of interleukin 6 during viral infections. Front. Microbiol. 10, 1057 (2019).
Jones, S. A. & Hunter, C. A. Is IL-6 a key cytokine target for therapy in COVID-19. Nat. Rev. Immunol. 21, 337–339 (2021).
Katze, M. G., He, Y. & Gale, M. Jr Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 (2002).
Chou, J., Thomas, P. G. & Randolph, A. G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 23, 177–185 (2022).
Li, M., Chen, L., Zhang, J., Xiong, C. & Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 15, e0230295 (2020).
Argueta, L. B. et al. SARS-CoV-2 infects syncytiotrophoblast and activates inflammatory responses in the placenta. Preprint at bioRxiv https://doi.org/10.1101/2021.06.01.446676 (2021).
Cribiu, F. M. et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J. Clin. Investig. https://doi.org/10.1172/JCI145427 (2021).
Bahadur, G. et al. Retrospective observational RT-PCR analyses on 688 babies born to 843 SARS-CoV-2 positive mothers, placental analyses and diagnostic analyses limitations suggest vertical transmission is possible. Facts Views Vis. Obgyn. 13, 53–66 (2021).
Flannery, D. D. et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 175, 594–600 (2021).
Boateng, J. O. et al. SARS-CoV-2 in infant urine and fecal samples after in utero COVID-19 exposure. Pediatr. Res. https://doi.org/10.1038/s41390-021-01822-x (2021).
Park, S. K. et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 19, 1387–1394.e1382 (2021).
Zhang, T. et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 92, 909–914 (2020).
Yamaji, M., Mahmoud, M., Evans, I. M. & Zachary, I. C. Neuropilin 1 is essential for gastrointestinal smooth muscle contractility and motility in aged mice. PLoS ONE 10, e0115563 (2015).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 (2012).
Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature 597, 250–255 (2021).
Ibrahim, S. H. et al. Liver diseases in the perinatal period: interactions between mother and infant. Hepatology 71, 1474–1485 (2020).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science https://doi.org/10.1126/science.abd4585 (2020).
Zuo, Y. et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abd3876 (2020).
Chang, S. E. et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun.12, 5417 (2021).
van der Wijst, M. G. P. et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abh2624 (2021).
Wu, Y. T. et al. Neonatal outcome in 29 pregnant women with COVID-19: A retrospective study in Wuhan, China. PLoS Med. 17, e1003195 (2020).
Mehl, S. C. et al. Necrotizing enterocolitis-like pneumatosis intestinalis in an infant with COVID-19. Pediatr. Infect. Dis. J. 40, e85–e86 (2021).
Investigators, R.-C. et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med. 384, 1491–1502 (2021).
Heuberger, J. et al. Epithelial response to IFN-gamma promotes SARS-CoV-2 infection. EMBO Mol. Med. 13, e13191 (2021).
Chen, L. Y. C., Hoiland, R. L., Stukas, S., Wellington, C. L. & Sekhon, M. S. Assessing the importance of interleukin-6 in COVID-19. Lancet Respir. Med. 9, e13 (2021).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644 (2021).
Sanidad, K. Z. & Zeng, M. Y. Neonatal gut microbiome and immunity. Curr. Opin. Microbiol. 56, 30–37 (2020).
Acknowledgements
We would like to thank all participants in this research study for their continued support of neonatal microbiome research.
Funding
This work was supported by NIH grant 5 K01 DK114376 and funds from the Gale and Ira Drukier Institute for Children’s Health and Children’s Health Council at Weill Cornell Medicine, the Center for Immunology and Office of Academic Integration of Cornell University, the Center for IBD Research at Weill Cornell Medicine, and the Hartwell Foundation (all to M.Y.Z.), a Hartwell Foundation Postdoctoral Fellowship (to K.Z.S.), a CTSC TL1 Scholar Award at Weill Cornell Medicine and the Biocodex Microbiota Foundation Grant (to J.A.B.) and the NCI R01CA234614, NIAID 2R01AI107301, and NIDDK R01DK121072 and 1RO3DK117252, Department of Medicine, Weill Cornell Medicine (to R.E.S.), and a Weill Cornell Medicine COVID-19 Research Grant (H.S., R.E.S., R.N.B.). R.E.S. is an Irma Hirschl Trust Research Award Scholar.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. M.Y.Z. conceived and supervised the study, analyzed data, and wrote the manuscript. J.C.J. collected stool specimens, performed experiments, analyzed data, and wrote the manuscript. J.M.P. helped with specimen collection and advised the study. A.A. and J.A.B. performed experiments and analyzed data. S.L.R. collected stool specimens and analyzed data. Y.B., K.Z.S., and M.A. assisted with experiments. R.N.B., H.S., and R.E. helped with analysis of placental tissues. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.Y.Z. is a consultant for Guidepoint. R.E.S. is on the scientific advisory board of Miromatrix Inc. and is a consultant and speaker for Alnylam Inc. The rest of the authors have no conflicts of interest to disclose.
Ethics approval and consent to participate
Parents of all subjects gave informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, J.C., Ananthanarayanan, A., Brown, J.A. et al. SARS CoV-2 detected in neonatal stool remote from maternal COVID-19 during pregnancy. Pediatr Res 93, 1375–1382 (2023). https://doi.org/10.1038/s41390-022-02266-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02266-7
This article is cited by
-
SARS-CoV-2 infection during pregnancy and necrotizing enterocolitis: case report and review of the literature
Italian Journal of Pediatrics (2025)
-
SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC)
Nature Immunology (2023)