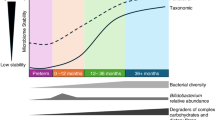
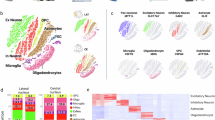
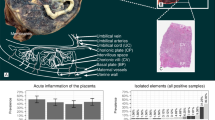
Abstract
Immunoperinatology is an emerging field. Transdisciplinary efforts by physicians, physician‐scientists, basic science researchers, and computational biologists have made substantial advancements by identifying unique immunologic signatures of specific diseases, discovering innovative preventative or treatment strategies, and establishing foundations for individualized neonatal intensive care of the most vulnerable neonates. In this review, we summarize the immunobiology and immunopathology of pregnancy, highlight omics approaches to study the maternal–fetal interface, and their contributions to pregnancy health. We examined the importance of transdisciplinary, multiomic (such as genomics, transcriptomics, proteomics, metabolomics, and immunomics) and machine-learning strategies in unraveling the mechanisms of adverse pregnancy, neonatal, and childhood outcomes and how they can guide the development of novel therapies to improve maternal and neonatal health.
Impact
-
Discuss immunoperinatology research from the lens of omics and machine-learning approaches.
-
Identify opportunities for omics-based approaches to delineate infection/inflammation-associated maternal, neonatal, and later life adverse outcomes (e.g., histologic chorioamnionitis [HCA]).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed for this review.
References
Humberg, A. et al. Preterm birth and sustained inflammation: consequences for the neonate. Semin. Immunopathol. 42, 451–468 (2020).
Peterson, L. S. et al. Multiomic immune clockworks of pregnancy. Semin. Immunopathol. 42, 397–412 (2020).
Robertson, S. A., Care, A. S. & Moldenhauer, L. M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 128, 4224–4235 (2018).
Wright, M. L., Starkweather, A. R. & York, T. P. Mechanisms of the maternal exposome and implications for health outcomes. ANS Adv. Nurs. Sci. 39, E17–E30 (2016).
Almond, D. & Currie, J. Killing me softly: the fetal origins hypothesis. J. Econ. Perspect. 25, 153–172 (2011).
Barker, D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990).
Carpinello, O. J., DeCherney, A. H. & Hill, M. J. Developmental origins of health and disease: the history of the barker hypothesis and assisted reproductive technology. Semin. Reprod. Med. 36, 177–182 (2018).
Wild, C. P. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 14, 1847–1850 (2005).
Raju, T. N. K., Buist, A. S., Blaisdell, C. J., Moxey-Mims, M. & Saigal, S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 106, 1409–1437 (2017).
Dover, G. J. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans. Am. Clin. Climatol. Assoc. 120, 199–207 (2009).
Rappaport, S. M., Barupal, D. K., Wishart, D., Vineis, P. & Scalbert, A. The blood exposome and its role in discovering causes of disease. Environ. Health Perspect. 122, 769–774 (2014).
Hasin, Y., Seldin, M. & Lusis, A. Multi-omics approaches to disease. Genome Biol. 18, 83 (2017).
Green, E. S. & Arck, P. C. Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus. Semin. Immunopathol. 42, 413–429 (2020).
Ozen, M. & Burd, I. Immunoperinatology. Am. J. Reprod. Immunol. 79, e12847 (2018).
Gomez-Chavez, F. et al. NF-kappaB and its regulators during pregnancy. Front. Immunol. 12, 679106 (2021).
Sakowicz, A. The role of NFkappaB in the three stages of pregnancy - implantation, maintenance, and labour: a review article. BJOG 125, 1379–1387 (2018).
Hadfield, K. A., McCracken, S. A., Ashton, A. W., Nguyen, T. G. & Morris, J. M. Regulated suppression of NF-kappaB throughout pregnancy maintains a favourable cytokine environment necessary for pregnancy success. J. Reprod. Immunol. 89, 1–9 (2011).
McCracken, S. A., Drury, C. L., Lee, H. S. & Morris, J. M. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J. Reprod. Immunol. 58, 27–47 (2003).
Toscano, M. A. et al. Nuclear factor (NF)-kappaB controls expression of the immunoregulatory glycan-binding protein galectin-1. Mol. Immunol. 48, 1940–1949 (2011).
McCracken, S. A., Gallery, E. & Morris, J. M. Pregnancy-specific down-regulation of NF-kappa B expression in T cells in humans is essential for the maintenance of the cytokine profile required for pregnancy success. J. Immunol. 172, 4583–4591 (2004).
McCracken, S. A., Hadfield, K., Rahimi, Z., Gallery, E. D. & Morris, J. M. NF-kappaB-regulated suppression of T-bet in T cells represses Th1 immune responses in pregnancy. Eur. J. Immunol. 37, 1386–1396 (2007).
Ross, K. M., Carroll, J. E., Dunkel Schetter, C., Hobel, C. & Cole, S. W. Pro-inflammatory immune cell gene expression during the third trimester of pregnancy is associated with shorter gestational length and lower birthweight. Am. J. Reprod. Immunol. 82, e13190 (2019).
Ariyakumar, G., Morris, J. M., McKelvey, K. J., Ashton, A. W. & McCracken, S. A. NF-kappaB regulation in maternal immunity during normal and IUGR pregnancies. Sci. Rep. 11, 20971 (2021).
Aghaeepour, N. et al. An immune clock of human pregnancy. Sci. Immunol. 2, eaan2946 (2017).
Aghaeepour, N. et al. A proteomic clock of human pregnancy. Am. J. Obstet. Gynecol. 218, 347 e341–347.e314 (2018).
Espinosa, C. et al. Data-driven modeling of pregnancy-related complications. Trends Mol. Med. 27, 762–776 (2021).
Fragiadakis, G. K. et al. Mapping the fetomaternal peripheral immune system at term pregnancy. J. Immunol. 197, 4482–4492 (2016).
Maric, I. et al. Early prediction of preeclampsia via machine learning. Am. J. Obstet. Gynecol. MFM 2, 100100 (2020).
Tarca, A. L. et al. Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep. Med. 2, 100323 (2021).
De Francesco, D. et al. A data-driven health index for neonatal morbidities. iScience 25, 104143 (2022).
De Francesco, D. et al. AI-driven longitudinal characterization of neonatal health and morbidity. Preprint at MedRxiv https://doi.org/10.1101/2022.03.31.22273233 (2022).
Tekola-Ayele, F. et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat. Commun. 13, 2384 (2022).
Stelzer, I. A. et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci. Transl. Med. 13, eabd9898 (2021).
West, L. J. Defining critical windows in the development of the human immune system. Hum. Exp. Toxicol. 21, 499–505 (2002).
Peterson, L. S. et al. Single-cell analysis of the neonatal immune system across the gestational age continuum. Front. Immunol. 12, 714090 (2021).
Sabic, D. & Koenig, J. M. A perfect storm: fetal inflammation and the developing immune system. Pediatr. Res. 87, 319–326 (2020).
Weitkamp, J. H. et al. Histological chorioamnionitis shapes the neonatal transcriptomic immune response. Early Hum. Dev. 98, 1–6 (2016).
Jackson, C. M. et al. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr. Res. 81, 384–390 (2017).
Rueda, C. M. et al. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum. Immunol. 76, 65–73 (2015).
Rychlik, K. A. & Sille, F. C. M. Environmental exposures during pregnancy: mechanistic effects on immunity. Birth Defects Res. 111, 178–196 (2019).
Kim, C. J., Romero, R., Chaemsaithong, P. & Kim, J. S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 213(4 Suppl), S53–S69 (2015).
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213(4 Suppl), S29–S52 (2015).
Romero, R. et al. The role of infection in preterm labour and delivery. Paediatr. Perinat. Epidemiol. 15(Suppl 2), 41–56 (2001).
Romero, R. et al. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 25, 21–39 (2007).
Gomez, R. et al. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 179, 194–202 (1998).
Yoon, B. H. et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am. J. Obstet. Gynecol. 174, 1433–1440 (1996).
Buck, C., Bundschu, J., Gallati, H., Bartmann, P. & Pohlandt, F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 93, 54–58 (1994).
Yoon, B. H. et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am. J. Obstet. Gynecol. 177, 406–411 (1997).
Kim, C. J. et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod. Pathol. 23, 1000–1011 (2010).
Kim, M. J. et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J. Immunol. 182, 3919–3927 (2009).
Lee, J. et al. Characterization of the fetal blood transcriptome and proteome in maternal anti-fetal rejection: evidence of a distinct and novel type of human fetal systemic inflammatory response. Am. J. Reprod. Immunol. 70, 265–284 (2013).
Hu, R. et al. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity 41, 605–619 (2014).
Ekiz, H. A. et al. T cell-expressed microRNA-155 reduces lifespan in a mouse model of age-related chronic inflammation. J. Immunol. 204, 2064–2075 (2020).
Bermick, J. R. et al. Neonatal monocytes exhibit a unique histone modification landscape. Clin. Epigenetics 8, 99 (2016).
Bermick, J. et al. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J. 286, 82–109 (2019).
Gomez-Lopez, N. et al. RNA sequencing reveals diverse functions of amniotic fluid neutrophils and monocytes/macrophages in intra-amniotic infection. J. Innate Immun. 13, 63–82 (2021).
Romero, R. et al. Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J. Matern. Fetal Neonatal Med. 23, 1344–1359 (2010).
Vicente-Munoz, S. et al. Vaginal metabolome: towards a minimally invasive diagnosis of microbial invasion of the amniotic cavity in women with preterm labor. Sci. Rep. 10, 5465 (2020).
Fattuoni, C. et al. Urinary metabolomic analysis to identify preterm neonates exposed to histological chorioamnionitis: a pilot study. PLoS ONE 12, e0189120 (2017).
Sureshchandra, S. et al. Single-cell RNA sequencing reveals immunological rewiring at the maternal-fetal interface following asymptomatic/mild SARS-CoV-2 infection. Cell Rep. 39, 110938 (2022).
Stafstrom, C. E. & Jantzie, L. L. COVID-19: Neurological considerations in neonates and children. Children 7, 133 (2020).
Shrestha, D. et al. Pulmonary immune cell transcriptome changes in double-hit model of BPD induced by chorioamnionitis and postnatal hyperoxia. Pediatr. Res. 90, 565–575 (2021).
Gayen Nee’ Betal, S. et al. Histological chorioamnionitis induces differential gene expression in human cord blood mononuclear leukocytes from term neonates. Sci. Rep. 9, 5862 (2019).
Alvira, C. M. & Morty, R. E. Can we understand the pathobiology of bronchopulmonary dysplasia? J. Pediatr. 190, 27–37 (2017).
Capasso, L. et al. Oxidative stress and bronchopulmonary dysplasia: evidences from microbiomics, metabolomics, and proteomics. Front. Pediatr. 7, 30 (2019).
Piersigilli, F. & Bhandari, V. Biomarkers in neonatology: the new “omics” of bronchopulmonary dysplasia. J. Matern. Fetal Neonatal Med. 29, 1758–1764 (2016).
Fabiano, A. et al. Metabolomic analysis of bronchoalveolar lavage fluid in preterm infants complicated by respiratory distress syndrome: preliminary results. J. Matern. Fetal Neonatal Med. 24(Suppl 2), 55–58 (2011).
Fanos, V. et al. Urinary metabolomics of bronchopulmonary dysplasia (BPD): preliminary data at birth suggest it is a congenital disease. J. Matern. Fetal Neonatal Med. 27(Suppl 2), 39–45 (2014).
Wheelock, C. E. et al. Application of ‘omics technologies to biomarker discovery in inflammatory lung diseases. Eur. Respir. J. 42, 802–825 (2013).
Carraro, S. et al. Airway metabolic anomalies in adolescents with bronchopulmonary dysplasia: new insights from the metabolomic approach. J. Pediatr. 166, 234–239 e231 (2015).
Oh, E. H., Song, H. S. & Park, T. H. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb. Technol. 48, 427–437 (2011).
Giambelluca, S. et al. Chorioamnionitis alters lung surfactant lipidome in newborns with respiratory distress syndrome. Pediatr. Res. 90, 1039–1043 (2021).
Watterberg, K. L., Demers, L. M., Scott, S. M. & Murphy, S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97, 210–215 (1996).
Dempsey, E., Chen, M. F., Kokottis, T., Vallerand, D. & Usher, R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am. J. Perinatol. 22, 155–159 (2005).
Lahra, M. M., Beeby, P. J. & Jeffery, H. E. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics 123, 1314–1319 (2009).
Lau, J. et al. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am. J. Obstet. Gynecol. 193, 708–713 (2005).
Ballard, A. R., Mallett, L. H., Pruszynski, J. E. & Cantey, J. B. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: a 25-year cohort. J. Perinatol. 36, 1045–1048 (2016).
Yoon, B. H., Park, C. W. & Chaiworapongsa, T. Intrauterine infection and the development of cerebral palsy. BJOG 110(Suppl 20), 124–127 (2003).
Bierstone, D. et al. Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatr. 172, 534–541 (2018).
Maisonneuve, E. et al. Association of chorioamnionitis with cerebral palsy at two years after spontaneous very preterm birth: the EPIPAGE-2 cohort study. J. Pediatr. 222, 71–78.e76 (2020).
Venkatesh, K. K. et al. Histologic chorioamnionitis and risk of neurodevelopmental impairment at age 10 years among extremely preterm infants born before 28 weeks of gestation. Am. J. Obstet. Gynecol. 223, 745.e741–745.e710 (2020).
Dudzik, D., Revello, R., Barbas, C. & Bartha, J. L. LC-MS-based metabolomics identification of novel biomarkers of chorioamnionitis and its associated perinatal neurological damage. J. Proteome Res. 14, 1432–1444 (2015).
Giussani, P., Prinetti, A. & Tringali, C. The role of Sphingolipids in myelination and myelin stability and their involvement in childhood and adult demyelinating disorders. J. Neurochem. 156, 403–414 (2021).
Chatterjee, S. & Pandey, A. The Yin and Yang of lactosylceramide metabolism: implications in cell function. Biochim. Biophys. Acta 1780, 370–382 (2008).
Bhunia, A. K., Arai, T., Bulkley, G. & Chatterjee, S. Lactosylceramide mediates tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J. Biol. Chem. 273, 34349–34357 (1998).
Won, J. S., Singh, A. K. & Singh, I. Lactosylceramide: a lipid second messenger in neuroinflammatory disease. J. Neurochem. 103(Suppl 1), 180–191 (2007).
Spiegel, A. M. et al. A genome-wide analysis of clinical chorioamnionitis among preterm infants. Am. J. Perinatol. 36, 1453–1458 (2019).
Hamilton, E. F. et al. Estimating risk of severe neonatal morbidity in preterm births under 32 weeks of gestation. J. Matern. Fetal Neonatal Med. 33, 73–80 (2020).
Park, Y. J. et al. Immune and inflammatory proteins in cord blood as predictive biomarkers of retinopathy of prematurity in preterm infants. Invest. Ophthalmol. Vis. Sci. 60, 3813–3820 (2019).
Danielsson, H. et al. Blood protein profiles related to preterm birth and retinopathy of prematurity. Pediatr. Res. 91, 937–946 (2021).
Culos, A. et al. Integration of mechanistic immunological knowledge into a machine learning pipeline improves predictions. Nat. Mach. Intell. 2, 619–628 (2020).
Reiss, J. D. et al. Perinatal infection, inflammation, preterm birth, and brain injury: a review with proposals for future investigations. Exp. Neurol. 351, 113988 (2022).
Hastie, T. & Tibshirani, R. & Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations. Monographs on Statistics and Applied Probability (CRC Press, 2015).
Kumar, N., Akangire, G., Sullivan, B., Fairchild, K. & Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatr. Res. 87, 210–220 (2020).
Fairchild, K. D. et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. J. Perinatol. 34, 375–379 (2014).
Sullivan, B. A., Grice, S. M., Lake, D. E., Moorman, J. R. & Fairchild, K. D. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J. Pediatr. 164, 775–780 (2014).
Tataranno, M. L., Vijlbrief, D. C., Dudink, J. & Benders, M. Precision medicine in neonates: a tailored approach to neonatal brain injury. Front. Pediatr. 9, 634092 (2021).
Acknowledgements
We thank Dr. Brice Gaudilliere, MD, PhD for his critical review of the manuscript. This work was supported in part by Johns Hopkins University School of Medicine Clinician-Scientist Award (JHUSOM CSA). NIH (R01HL139492 and R35GM138353), Burroughs Wellcome Fund (1019816), Robertson Foundation, Christopher Hess Research Fund, the Alfred E. Mann Foundation, the March of Dimes, and the Bill and Melinda Gates Foundation (INV-001734, OPP1113682, INV-003225, INV037517).
Author information
Authors and Affiliations
Contributions
M.O., N.A., I.M., R.J.W., D.K.S., and L.L.J. contributed to the writing and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No patient consent was required for this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozen, M., Aghaeepour, N., Marić, I. et al. Omics approaches: interactions at the maternal–fetal interface and origins of child health and disease. Pediatr Res 93, 366–375 (2023). https://doi.org/10.1038/s41390-022-02335-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02335-x
This article is cited by
-
Cord blood metabolomics: a window into future heart health
Pediatric Research (2025)
-
Proteomic studies of VEGFR2 in human placentas reveal protein associations with preeclampsia, diabetes, gravidity, and labor
Cell Communication and Signaling (2024)
-
Emerging role of artificial intelligence, big data analysis and precision medicine in pediatrics
Pediatric Research (2023)