Abstract
Background
Preclinical data demonstrate that opioids modulate brain reward signaling through an inflammatory cascade, but this relationship has yet to be studied in opioid-exposed neonates.
Methods
Saliva samples of 54 opioid-exposed and sex- and age-matched non-exposed neonates underwent transcriptomic analysis of inflammatory and reward genes. A subset of 22 neonates underwent brain magnetic resonance imaging (MRI) to evaluate white matter injury commonly associated with inflammatory response. Gene expression and brain MRI were compared between opioid- and non-exposed neonates and further stratified by sex and pharmacotherapy need.
Results
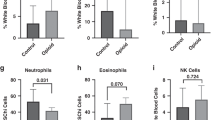
Opioid-exposed females regardless of pharmacotherapy need had higher expression of inflammatory genes than their male counterparts, with notable differences in the expression of CCL2 and CXCL1 in females requiring pharmacotherapy (p = 0.01 and 0.06, respectively). Opioid-exposed males requiring pharmacotherapy had higher expression of DRD2 than exposed females (p = 0.07), validating our prior research. Higher expression of IL1β, IL6, TNFα, and IL10 was seen in opioid-exposed neonates with T1 white matter hyperintensity (WMH) compared to exposed neonates without WMH (p < 0.05).
Conclusion
Prenatal opioid exposure may promote inflammation resulting in changes in reward signaling and white matter injury in the developing brain, with unique sex-specific effects. The actions of opioids through non-neuronal pathways need further investigation.
Impact
-
Opioid-exposed neonates are at risk for punctate T1 white matter hyperintensity (WMH).
-
Females carry a greater propensity for WMH.
-
Salivary transcriptomic data showed significantly higher expression of inflammatory genes in opioid-exposed neonates with WMH than those without WMH, irrespective of pharmacotherapy need.
-
Adding to prior studies, our findings suggest that prenatal opioid exposure may modulate white matter injury and reward signaling through a pro-inflammatory process that is sex specific.
-
This novel study highlights the short-term molecular and structural effects of prenatal opioids and the need to elucidate the long-term impact of prenatal opioid exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Materials described in the manuscript, including all relevant raw data, are available for research and non-commercial purposes upon reasonable request, without breaching participant confidentiality.
References
Honein, M. A., Boyle, C. & Redfield, R. R. Public health surveillance of prenatal opioid exposure in mothers and neonates. Pediatrics 143, e20183801 (2019).
Kelty, E. & Preen, D. B. Risk factors associated with the occurrence of neonatal opioid withdrawal syndrome: a review. CNS Drugs 33, 1113–1120 (2019).
Isemann, B. T., Stoeckle, E. C., Taleghani, A. A. & Mueller, E. W. Early prediction tool to identify the need for pharmacotherapy in neonates at risk for neonatal abstinence syndrome. Pharmacother 37, 840–848 (2017).
Wachman, E. M. et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 309, 1821–1827 (2013).
Cole, F. S., Wegner, D. J. & Davis, J. M. The genomics of neonatal abstinence syndrome. Front. Pediatr. 5, 176 (2017).
Wang, X. et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl Acad. Sci. USA 109, 6325–6330 (2012).
Bland, S. T., Hutchinson, M. R., Maier, S. F., Watkins, L. R. & Johnson, K. W. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav. Immun. 23, 492–497 (2009).
Hutchinson, M. R. et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci. 32, 11187–11200 (2012).
Lacagnina, M. J., Rivera, P. D. & Bilbo, S. D. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharm 42, 156–177 (2017).
Kashima, D. T. & Grueter, B. A. Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc. Natl Acad. Sci. USA 114, 8865–8870 (2017).
Zhang, H., Largent-Milnes, T. M. & Vanderal, T. W. Glial neuroimmune signaling in opioid reward. Brain. Res. Bull. 155, 102–110 (2020).
Jantzie, L. L. et al. Prenatal opioid exposure: the next neuroinflammatory disease. Brain Behav. Immun. 84, 45–58 (2020).
Monnelly, V. J. et al. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin. 18, 9–14 (2017).
Merhar, S. L. et al. White matter injury and structural anomalies in infants with prenatal opioid exposure. AJNR Am. J. Neuroradiol. 40, 2161–2165 (2019).
Saijo, K. & Glass, C. K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11, 775–787 (2011).
Ginhoux, F. & Prinz, M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 7, a020537 (2015).
Jin, C., Londono, I., Mallard, C. & Lodygensky, G. A. New means to assess neonatal inflammatory injury. J. Neuroinflammation 12, 180 (2015).
Leviton, A. et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr. 158, 897–903 (2011).
Zhan, D. et al. Intrauterine inflammation induced white matter injury protection by fibrinogen-like protein 2 deficiency in perinatal mice. Pediatr. Res. 89, 1706–1714 (2021).
Yen, E. et al. Sex-dependent gene expression in infants with neonatal opioid withdrawal syndrome. J. Pediatr. 214, 60–65 (2019).
Pace, N. P. & Vassallo, J. Association between neutrophil-lymphocyte ratio and gestational diabetes—a systematic review and meta analysis. J. Endo. Soc. 5, 1–11 (2021).
Cappelletti, M., Presicce, P. & Kallapur, S. G. Immunobiology of acute chorioamnionitis. Front. Immunol. 11, 649 (2020).
Baloch, R. Q., Pinto, J. M., Greenberg, P., Kuo, Y.-H. & Siu, A. Evaluation and analysis of modified Finnegan scoring system for assessment of neonatal abstinence syndrome. J. Opioid. Manag. 16, 189–196 (2020).
Dietz, J. A., Johnson, K. L., Wick, H. C., Bianchi, D. W. & Maron, J. L. Optimal techniques for mRNA extraction from neonatal salivary supernatant. Neonatology 101, 55–60 (2012).
Yen, E., Kaneko-Tarui, T. & Maron, J. L. Technical considerations and protocol optimization for neonatal salivary biomarker discovery and analysis. Front. Pediatr. 8, 618553 (2021).
Ramesh, G., MacLean, A. G. & Philipp, M. T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013, 480739 (2013).
Bachtell, R. et al. Targeting the toll of drug abuse: the translational potential of toll-like receptor 4. CNS Neurol. Disord. Drug Targets 14, 692–699 (2015).
Cui, C., Shurtleff, D. & Harris, R. A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 118, 1–12 (2014).
Khanna, P., Johnson, K. L. & Maron, J. L. Optimal reference genes for RT-qPCR normalization in the newborn. Biotech. Histochem. 92, 459–466 (2017).
Korom, M. et al. Dear reviewers: responses to common reviewer critiques about infant neuroimaging studies. Dev. Cogn. Neurosci. 53, 101055 (2022).
Buyanova, I. S. & Arsalidou, M. Cerebral white matter myelination and relations to age, gender, and cognition: a selective review. Front. Hum. Neurosci. 15, 662031 (2021).
Stevens, C. W., Aravind, S., Das, S. & Davis, R. L. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br. J. Pharmacol. 168, 1421–1429 (2013).
Zhang, P. et al. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front. Immunol. 11, 1456 (2020).
Hutchinson, M. R. et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 24, 83–95 (2010).
Yirmiya, R. & Goshen, I. Immune modulation of learning, memory, neural plasticity, and neurogenesis. Brain Behav. Immun. 25, 181–213 (2011).
Cant, R., Dalgleish, A. G. & Allen, R. L. Naltrexone inhibits IL-6 and TNFα production in human immune cell subsets following stimulation with ligands for intracellular toll-like receptors. Front. Immunol. 8, 809 (2017).
Sun, Y., Chen, G., Zhou, K. & Zhu, Y. A conditioned place preference protocol for measuring incubation of craving in rats. J. Vis. Exp. 141, e58384 (2018).
Volkow, N. D., Fowler, J. S., Wang, G.-J. & Swanson, J. M. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557–569 (2004).
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible confounding factors. NeuroImage 42, 1537–1543 (2008).
Charles, M. K. et al. Male sex associated with increased risk of neonatal abstinence syndrome. Hosp. Pediatr. 7, 328–334 (2017).
Silver, E. R. & Hur, C. Gender differences in prescription opioid use and misuse: implications for men’s health and the opioid epidemic. Prev. Med. 131, 105946 (2020).
Jeanne, M. et al. Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J. Addict. Med. 9, 46–52 (2015).
Beagley, S. M. et al. Incidence and characteristics of non-fatal opioid overdose among youths aged 11 to 24 years by sex. JAMA Netw. Open 3, e2030201 (2020).
Kennedy, A. P., Epstein, D. H., Phillips, K. A. & Preston, K. L. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 132, 29–37 (2013).
Graeve, R. et al. Infants’ prenatal exposure to opioids and the association with birth outcomes: a systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 36, 125–143 (2022).
Azuine, R. E. et al. Prenatal risk factors and perinatal and postnatal outcomes associated with maternal opioid exposure in an urban, low-income, multiethnic US population. JAMA Netw. Open. 2, e196405 (2019).
Leyenaar, J. K. et al. Infant mortality associated with prenatal opioid exposure. JAMA Pediatr. 175, 706–714 (2021).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 134, 331–349 (2017).
Back, S. A. & Rosenberg, P. A. Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815 (2015).
Hayman, M. et al. Punctate white-matter lesions in the full-term newborn: underlying aetiology and outcome. Eur. J. Paed. Neurol. 23, 280–287 (2019).
Alaee, A., Zarghami, M., Farnia, S., Khademloo, M. & Khoddad, T. Comparison of brain white matter hyperintensities in methamphetamine and methadone dependent patients and healthy controls. Iran. J. Radiol. 11, e14275 (2014).
Kidokoro, H., Anderson, P. J., Doyle, L. W., Neil, J. J. & Inder, T. E. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am. J. Neuroradiol. 32, 2005–2010 (2011).
Guo, T. et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurol 88, 614–622 (2017).
Galinsky, R. et al. Magnetic resonance imaging correlates of white matter gliosis and injury in preterm fetal sheep exposed to progressive systemic inflammation. Int. J. Mol. Sci. 21, 8891 (2020).
Leviton, A. & Dammann, O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr. Res. 55, 542–545 (2004).
Gilles, F. H. & Leviton, A. Neonatal white matter damage and the fetal inflammatory response. Semin. Fetal Neonatal Med. 25, 101111 (2020).
Ruwanpathirana, R. et al. Prematurity reduces the severity and need for treatment of neonatal abstinence syndrome. Acta Paediatr. 104, e188–e194 (2015).
Oakes, L. M. Sample size, statistical power, and false conclusions in infant looking-time research. Infancy 22, 436–439 (2017).
Lausten-Thomsen, U., Olsen, M., Greisen, G. & Schmiegelow, K. Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr. Pathol. 33, 114–118 (2014).
Yepes-Calderon, F. et al. Tractography in the clinics: implementing a pipeline to characterize early brain development. NeuroImage Clin. 14, 629–640 (2017).
Acknowledgements
We would like to thank all the families for participating in this study. We also thank the entire staff at the Tufts Shields MRI for their assistance in organizing and acquiring the images.
Funding
This study received funding from K12 Building Interdisciplinary Research for Careers in Women’s Health (BIRCWH) Grant #5K12HD092535-05, Charles H. Hood Foundation Child Health Research Grant, and Tufts Tiny Feet Funds. The project was supported by the National Center for Advancing Translational Sciences, NIH, #UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
Conception and design: E.Y., N.M., T.T., T.K.-T., J.L.M. Acquisition of data: E.Y., N.M. Analysis and interpretation of data: E.Y., J.L.B. Drafting the article: E.Y. Critical revision: E.Y., N.M., T.T., T.K.-T., J.L.B., J.M.D., J.L.M. Final approval of the version to be published: E.Y., N.M., T.T., T.K.-T., J.L.B., J.M.D., J.L.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all subjects enrolled in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yen, E., Madan, N., Tarui, T. et al. Sex-specific inflammatory and white matter effects of prenatal opioid exposure: a pilot study. Pediatr Res 93, 604–611 (2023). https://doi.org/10.1038/s41390-022-02357-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02357-5
This article is cited by
-
Efficacy of stochastic vibro-tactile stimulation for newborns at risk of neonatal opioid withdrawal syndrome
Pediatric Research (2025)
-
Severity of punctate white matter lesions in preterm infants: antecedents and cerebral palsy prediction
Pediatric Research (2025)