Abstract
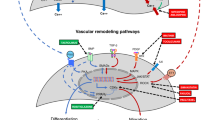
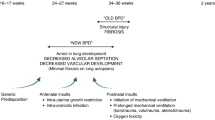
Bronchopulmonary dysplasia (BPD) is the most common complication of preterm birth. Up to 1/3 of children with BPD develop pulmonary hypertension (PH). PH increases mortality, the risk of adverse neurodevelopmental outcome and lacks effective treatment. Current vasodilator therapies address symptoms, but not the underlying arrested vascular development. Recent insights into placental biology and novel technological advances enabling the study of normal and impaired lung development at the single cell level support the concept of a vascular phenotype of BPD. Dysregulation of growth factor pathways results in depletion and dysfunction of putative distal pulmonary endothelial progenitor cells including Cap1, Cap2, and endothelial colony-forming cells (ECFCs), a subset of vascular progenitor cells with self-renewal and de novo angiogenic capacity. Preclinical data demonstrate effectiveness of ECFCs and ECFC-derived particles including extracellular vesicles (EVs) in promoting lung vascular growth and reversing PH, but the mechanism is unknown. The lack of engraftment suggests a paracrine mode of action mediated by EVs that contain miRNA. Aberrant miRNA signaling contributes to arrested pulmonary vascular development, hence using EV- and miRNA-based therapies is a promising strategy to prevent the development of BPD-PH. More needs to be learned about disrupted pathways, timing of intervention, and mode of delivery.
Impact
-
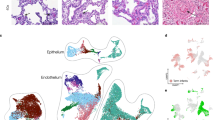
Single-cell RNA sequencing studies provide new in-depth view of developmental endothelial depletion underlying BPD-PH.
-
Aberrant miRNA expression is a major cause of arrested pulmonary development.
-
EV- and miRNA-based therapies are very promising therapeutic strategies to improve prognosis in BPD-PH.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Data sharing is not applicable as no datasets were generated or analyzed for this review.
References
Thébaud, B. et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers. 5, 1–23 (2019).
Stoll, B. J. et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Jobe, A. H., Bancalari, E. Bronchopulmonary dysplasia. In: Am. J. Respir. Crit. Care Med. Vol 163. American Lung Association; 2001:1723-1729.
Arjaans, S. et al. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 32, 258–267 (2018).
Nagiub, M., Kanaan, U., Simon, D. & Guglani, L. Risk factors for development of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review and meta-analysis. Paediatr. Respir. Rev. 23, 27–32 (2017).
Khemani, E. et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: Clinical features and outcomes in the surfactant era. Pediatrics 120, 1260–1269 (2007).
Barrington, K. J., Finer, N., Pennaforte, T., Altit, G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2017, CD000399 (2017).
Stritzke, A., Bhandari, V. & Lodha, A. Use of inhaled nitric oxide in preterm infants: is there sufficient evidence? Indian J. Pediatr. 89, 262–266 (2022).
Mourani, P. M., Sontag, M. K., Ivy, D. D. & Abman, S. H. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J. Pediatr. 154, 379–384 (2009).
Nyp, M., Sandritter, T., Poppinga, N., Simon, C. & Truog, W. E. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: Any benefits? J. Perinatol. 32, 64–69 (2012).
Tan, K., Krishnamurthy, M. B., O’Heney, J. L., Paul, E. & Sehgal, A. Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. Eur. J. Pediatr. 174, 1109–1115 (2015).
Trottier-Boucher, M. N. et al. Sildenafil for the treatment of pulmonary arterial hypertension in infants with bronchopulmonary dysplasia. Pediatr. Cardiol. 36, 1255–1260 (2015).
Jackson, W. et al. Safety of sildenafil in extremely premature infants: a phase I trial. J. Perinatol. 42, 31–36 (2022).
Katsuragi, S. et al. Riociguat can ameliorate bronchopulmonary dysplasia in the SU5416 induced rat experimental model. Exp. Lung Res. 47, 382–389 (2021).
Donda, K. et al. Riociguat prevents hyperoxia-induced lung injury and pulmonary hypertension in neonatal rats without effects on long bone growth. PLoS One 13, e0199927 (2018).
Jeremiasen, I., Naumburg, E., Westöö, C. G., Weismann, C. & Tran-Lundmark, K. Vasodilator therapy for pulmonary hypertension in children: a national study of patient characteristics and current treatment strategies. Pulm Circ. 11, 20458940211057891 (2021).
Spreemann, T., Bertram, H., Happel, C. M., Kozlik-Feldmann, R. & Hansmann, G. First-in-child use of the oral soluble guanylate cyclase stimulator riociguat in pulmonary arterial hypertension. Pulm Circ. 8, 2045893217743123 (2018).
Rugolotto, S. et al. Weaning of epoprostenol in a small infant receiving concomitant bosentan for severe pulmonary arterial hypertension secondary to bronchopulmonary dysplasia. Minerva Pediatr. 58, 491–494 (2006).
Gürakan, B., Kayiran, P., Öztürk, N., Kayiran, S. M. & Dindar, A. Therapeutic combination of sildenafil and iloprost in a preterm neonate with pulmonary hypertension. Pediatr. Pulmonol. 46, 617–620 (2011).
Hwang, S. K., O, Y. C., Kim, N. S., Park, H. K. & Yum, M. K. Use of inhaled iloprost in an infant with bronchopulmonary dysplasia and pulmonary artery hypertension. Korean Circ. J. 39, 342–345. (2009).
Piastra, M. et al. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr. Pulmonol. 47, 757–762 (2012).
Ferdman, D. J., Rosenzweig, E. B., Zuckerman, W. A. & Krishnan, U. Subcutaneous treprostinil for pulmonary hypertension in chronic lung disease of infancy. Pediatrics 134, e274–e278 (2014).
Kadmon, G. et al. Pulmonary hypertension specific treatment in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 52, 77–83 (2017).
Migdał, A. et al. Children with bronchopulmonary dysplasia-associated pulmonary hypertension treated with pulmonary vasodilators-the pediatric cardiologist point of view. Children (Basel) 8, 326–338 (2021).
Le Cras, T. D., Markham, N. E., Tuder, R. M., Voelkel, N. F. & Abman, S. H. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555–L562 (2002). 3.
Nicolls, M. R. et al. New models of pulmonary hypertension based on VEGF receptor blockade-induced endothelial cell apoptosis. Pulm. Circ. 2, 434–442 (2012).
Kunig, A. M. et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L529–L535 (2005). 4.
Thébaud, B. et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: Evidence that angiogenesis participates in alveolarization. Circulation 112, 2477–2486 (2005).
Vila Ellis, L. et al. Epithelial vegfa specifies a distinct endothelial population in the mouse lung. Dev. Cell 52, 617–630.e6 (2020).
Orriols, M., Gomez-Puerto, M. C. & ten Dijke, P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell. Mol. Life Sci. 74, 2979–2995 (2017).
Garcia-Rivas, G., Jerjes-Sánchez, C., Rodriguez, D., Garcia-Pelaez, J. & Trevino, V. A systematic review of genetic mutations in pulmonary arterial hypertension. BMC Med. Genet 18, 82 (2017).
Yee, M. et al. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am. J. Pathol. 178, 2601–2610 (2011).
Arjaans, X. S. et al. Early angiogenic proteins associated with high risk for bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Am. J. Physiol. Lung Cell Mol. Physiol. 318, L644–L654 (2020).
Heydarian, M. et al. Relationship between impaired BMP signalling and clinical risk factors at early-stage vascular injury in the preterm infant. Thorax. Published online May 17, 2022:thoraxjnl-2021-218083.
Gauldie, J. et al. Transfer of the active form of transforming growth factor-β1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am. J. Pathol. 163, 2575–2584 (2003).
Le Saux, O. et al. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L1007–L1017 (2008).
Fan, W. H., Pech, M. & Karnovsky, M. J. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur. J. Cell Biol. 79, 915–923 (2000).
Thomas, W. et al. Airway concentrations of angiopoietin-1 and endostatin in ventilated extremely premature infants are decreased after funisitis and unbalanced with bronchopulmonary dysplasia/death. Pediatr. Res. 65, 468–473 (2009).
Mohamed, W. A. W., Niyazy, W. H. & Mahfouz, A. A. Angiopoietin-1 and endostatin levels in cord plasma predict the development of bronchopulmonary dysplasia in preterm infants. J. Trop. Pediatr. 57, 385–388 (2011).
Kim, D. H. & Kim, H. S. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology 106, 55–61 (2014).
Lai, S. H. et al. Low cord-serum 25-hydroxyvitamin D levels are associated with poor lung function performance and increased respiratory infection in infancy. PLoS One 12, e0173268 (2017).
Foong, R. E. et al. The effects of in utero Vitamin D deficiency on airway smooth muscle mass and lung function. Am. J. Respir. Cell Mol. Biol. 53, 664–675 (2015).
Wang, Y. & Jiang, L. Role of vitamin D-vitamin D receptor signaling on hyperoxia-induced bronchopulmonary dysplasia in neonatal rats. Pediatr. Pulmonol. 56, 2335–2344 (2021).
Groenman, F. A. et al. Effect of chemical stabilizers of hypoxia-inducible factors on early lung development. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L557–L567 (2007).
Shimoda, L. A. & Semenza, G. L. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am. J. Respir. Crit. Care Med. 183, 152–156 (2011).
Peplow, P. V. Influence of growth factors and cytokines on angiogenic function of endothelial progenitor cells: a review of in vitro human studies. Growth Factors 32, 83–116 (2014).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Ingram, D. A. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 (2004).
Alvarez, D. F. et al. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L419–L430 (2008).
Balasubramaniam, V., Mervis, C. F., Maxey, A. M., Markham, N. E. & Abman, S. H. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: Implications for the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1073–L1084 (2007).
Baker, C. D. et al. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am. J. Respir. Crit. Care Med. 180, 454–461 (2009).
Baker, C. D. et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur. Respir. J. 40, 1516–1522 (2012).
Alphonse, R. S. et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 129, 2144–2157 (2014).
Ren, X. et al. Postnatal alveologenesis depends on FOXF1 signaling in c-KIT1 endothelial progenitor cells. Am. J. Respir. Crit. Care Med. 200, 1164–1176 (2019).
Muñoz-Hernandez, R. et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension 64, 165–171 (2014).
Gumina, D. L., Black, C. P., Balasubramaniam, V., Winn, V. D. & Baker, C. D. Umbilical cord blood circulating progenitor cells and endothelial colony-forming cells are decreased in preeclampsia. Reprod. Sci. 24, 1088–1096 (2017).
Schröder‐Heurich, B. et al. Downregulation of miR‐1270 mediates endothelial progenitor cell function in preeclampsia: Role for ATM in the Src/VE‐cadherin axis. FASEB J. 36 (2022).
Sipos, P. I. et al. Endothelial colony-forming cells derived from pregnancies complicated by intrauterine growth restriction are fewer and have reduced vasculogenic capacity. J. Clin. Endocrinol. Metab. 98, 4953–4960 (2013).
Gillich, A. et al. Capillary cell-type specialization in the alveolus. Nature 586, 785–789 (2020).
Negretti, N. M. et al. A single-cell atlas of mouse lung development. Development (Cambridge) 148, dev199512 (2021).
Hurskainen, M. et al. Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat. Commun. 12, 1565 (2021).
Sun, X. et al. A census of the lung: CellCards from LungMAP. Dev. Cell. 57, 112–145.e2 (2022).
Sarlon, G. et al. Therapeutic effect of fucoidan-stimulated endothelial colony-forming cells in peripheral ischemia. J. Thromb. Haemost. 10, 38–48 (2012).
Goto, K. et al. Intravenous administration of endothelial colony-forming cells overexpressing integrin β 1 augments angiogenesis in ischemic legs. Stem Cells Transl. Med 5, 218–226 (2016).
Tan, Q. et al. Transplantation of healthy but not diabetic outgrowth endothelial cells could rescue ischemic myocardium in diabetic rabbits. Scand. J. Clin. Lab. Invest. 70, 313–321 (2010).
Burger, D. et al. Human endothelial colony-forming cells protect against acute kidney injury role of exosomes. Am. J. Pathol. 185, 2309–2323 (2015).
Xia, W. H. et al. BMP4/Id2 signaling pathway is a novel therapeutic target for late outgrowth endothelial progenitor cell-mediated endothelial injury repair. Int. J. Cardiol. 228, 796–804 (2017).
Baker, C. D. et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 305, L73–L81 (2013).
Liao, G., Zheng, K., Shorr, R. & Allan, D. S. Human endothelial colony-forming cells in regenerative therapy: A systematic review of controlled preclinical animal studies. Stem Cells Transl. Med 9, 1344–1352 (2020).
Prasain, N. et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 32, 1151–1157 (2014).
Wang, G. et al. Generation of pulmonary endothelial progenitor cells for cell-based therapy using interspecies mouse-rat chimeras. Am. J. Respir. Crit. Care Med. 204, 326–338 (2021).
Calcaterra, F. et al. Increased frequency and vasculogenic potential of endothelial colony-forming cells in patients with Kaposi’s sarcoma. J. Invest. Dermatol. 137, 1533–1540 (2017).
Campanelli, R. et al. Kinetic and angiogenic activity of circulating endothelial colony forming cells in patients with infantile haemangioma receiving propranolol. Thromb. Haemost. 119, 274–284 (2019).
Medina, R. J. et al. Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Transl. Med 6, 1316–1320 (2017).
Salybekov, A. A., Kobayashi, S. & Asahara, T. Characterization of endothelial progenitor cell: past, present, and future. Int. J. Mol. Sci. 23, 7697 (2022).
Zhang, X. et al. Exosomes secreted by endothelial progenitor cells improve the bioactivity of pulmonary microvascular endothelial cells exposed to hyperoxia in vitro. Ann. Transl. Med. 7, 254–254 (2019).
Zhong, X. Q. et al. Umbilical cord blood-derived exosomes from very preterm infants with bronchopulmonary dysplasia impaired endothelial angiogenesis: roles of exosomal MicroRNAs. Front. Cell Dev. Biol. 9, 637248 (2021).
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Wu, Y. T. et al. MicroRNA expression aberration associated with bronchopulmonary dysplasia in preterm infants: A preliminary study. Respir. Care 58, 1527–1535 (2013).
Zhang, X. et al. MicroRNA expression profile in hyperoxia-exposed newborn mice during the development of bronchopulmonary dysplasia. Respir. Care 56, 1009–1015 (2011).
Bhaskaran, M. et al. Identification of microRNAs changed in the neonatal lungs in response to hyperoxia exposure. Physiol. Genomics. 44, 970–980 (2012).
Olave, N. et al. Regulation of alveolar septation by microRNA-489. Am. J. Physiol. Lung Cell Mol. Physiol. 310, L476–L487 (2016).
Rogers, L. K. et al. Attenuation of MIR-17∼92 cluster in bronchopulmonary dysplasia. Ann. Am. Thorac. Soc. 12, 1506–1513 (2015).
Ruiz‐Camp, J. et al. Targeting miR‐34a/ Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia. EMBO Mol Med. 11, e9448 (2019).
Alam, M. A., Betal, S. G. N., Aghai, Z. H. & Bhandari, V. Hyperoxia causes miR199a-5p-mediated injury in the developing lung. Pediatr. Res 86, 579–588 (2019).
Chao, C. M. et al. Failure to down-regulate miR-154 expression in early postnatal mouse lung epithelium suppresses alveologenesis, with changes in Tgf-β signaling similar to those induced by exposure to hyperoxia. Cells 9, 849 (2020).
Yuan, H. S., Xiong, D. Q., Huang, F., Cui, J. & Luo, H. MicroRNA-421 inhibition alleviates bronchopulmonary dysplasia in a mouse model via targeting Fgf10. J. Cell. Biochem. 120, 16876–16887 (2019).
Gong, X., Qiu, J., Qiu, G. & Cai, C. Adrenomedullin regulated by miRNA-574-3p protects premature infants with bronchopulmonary dysplasia. Biosci. Rep. 40, BSR20191879 (2020).
Hu, Y. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol. Med. 26, 1–12 (2019).
Syed, M. et al. Hyperoxia causes MIR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat. Commun. 8, 1–17 (2017).
Cheng, H. et al. Knockdown of miR-203a-3p alleviates the development of bronchopulmonary dysplasia partly via the up-regulation of vascular endothelial growth factor A. J. Bioenerg. Biomembr. 53, 13–23 (2021).
Gilfillan, M., Das, P., Shah, D., Alam, M. A., Bhandari, V. Inhibition of microRNA-451 is associated with increased expression of Macrophage Migration Inhibitory Factor and mitigation of the cardio-pulmonary phenotype in a murine model of Bronchopulmonary Dysplasia. Respir. Res. 21 (2020).
Freeman, A. et al. MicroRNA 219-5p inhibits alveolarization by reducing platelet derived growth factor receptor-alpha. Respir. Res. 22, 1–9 (2021).
Zhang, J. et al. Exploring cell-specific miRNA regulation with single-cell miRNA-mRNA co-sequencing data. BMC Bioinform 22, 578 (2021).
Chakraborty, C., Bhattacharya, M. & Agoramoorthy, G. Single-cell sequencing of miRNAs: A modified technology. Cell Biol. Int. 44, 1773–1780 (2020).
Mukherjee, D. et al. Fetal pulmonary hypertension: dysregulated microRNA-34c-Notch1 axis contributes to impaired angiogenesis in an ovine model. Pediatr. Res. Published online 2022.
Wang, C. et al. Integrated MicroRNA-mRNA analyses of distinct expression profiles in hyperoxia-induced bronchopulmonary dysplasia in neonatal mice. Am J Perinatol. Published online 2021.
Funding
W.D. is financially supported by the Bekker Programme from the Polish National Agency for Academic Exchange – (Number BPN/BEK/2021/1/00329/ U/00001). B.T. is supported by the Canadian Institutes of Health Research (CIHR) and the Stem Cell Network.
Author information
Authors and Affiliations
Contributions
Concept and design: W.D. and B.T.; interpretation of relevant literature: W.D.; drafting the manuscript: W.D.; review and final approval of the manuscript: B.T. All authors have read and agreed to the published version of the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Durlak, W., Thébaud, B. The vascular phenotype of BPD: new basic science insights—new precision medicine approaches. Pediatr Res 96, 1162–1171 (2024). https://doi.org/10.1038/s41390-022-02428-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02428-7
This article is cited by
-
Establishment of a neonatal rat model of sequential hyperoxic hypoxia to recapitulate clinical progression of bronchopulmonary dysplasia-associated pulmonary hypertension
Intensive Care Medicine Experimental (2025)
-
Mitochondrial quality control mechanisms as molecular targets for impaired lung development: from fetuses to neonates
Respiratory Research (2025)
-
Sex differences in the risk of bronchopulmonary dysplasia and pulmonary hypertension: a Bayesian meta-analysis
Pediatric Research (2025)
-
Reinitiating lung development: a novel approach in the management of bronchopulmonary dysplasia
Respiratory Research (2024)
-
Adequate nutrition for bronchopulmonary dysplasia, but do not forget oxygen
Pediatric Research (2024)