Abstract
Background
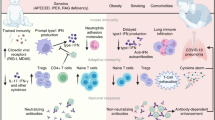
Although most children experience mild symptoms during acute SARS-CoV-2 infection, some develop the severe post-COVID-19 complication, Multisystem Inflammatory Syndrome in Children (MIS-C). While acute presentations of COVID-19 and MIS-C have been well immunophenotyped, little is known about the lasting immune profile in children after acute illness.
Methods
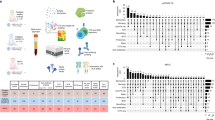
Children 2 months–20 years of age presenting with either acute COVID-19 (n = 9) or MIS-C (n = 12) were enrolled in a Pediatric COVID-19 Biorepository at a single medical center. We deeply profiled humoral immune responses and circulating cytokines following pediatric COVID-19 and MIS-C.
Results
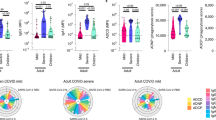
Twenty-one children and young adults provided blood samples at both acute presentation and 6-month follow-up (mean: 6.5 months; standard deviation: 1.77 months). Pro-inflammatory cytokine elevations resolved after both acute COVID-19 and MIS-C. Humoral profiles continue to mature after acute COVID-19, displaying decreasing IgM and increasing IgG over time, as well as stronger effector functions, including antibody-dependent monocyte activation. In contrast, MIS-C immune signatures, especially anti-Spike IgG1, diminished over time.
Conclusions
Here, we show the mature immune signature after pediatric COVID-19 and MIS-C, displaying resolving inflammation with recalibration of the humoral responses. These humoral profiles highlight immune activation and vulnerabilities over time in these pediatric post-infectious cohorts.
Impact
-
The pediatric immune profile matures after both COVID-19 and MIS-C, suggesting a diversified anti-SARS-CoV-2 antibody response after resolution of acute illness.
-
While pro-inflammatory cytokine responses resolve in the months following acute infection in both conditions, antibody-activated responses remain relatively heightened in convalescent COVID-19.
-
These data may inform long-term immunoprotection from reinfection in children with past SARS-CoV-2 infections or MIS-C.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All relevant data are included in this manuscript. Data is available upon reasonable request.
References
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Stephenson, T. et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open 11, e052838 (2021).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107 (2022).
Buonsenso, D. et al. Preliminary evidence on long COVID in children. Acta Paediatr. 110, 2208–2211 (2021).
Sacco, K. et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 28, 1050–1062 (2022).
Yonker, L. M. et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J. Pediatr. 227, 45.e5–52.e5 (2020).
Bartsch, Y. C. et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 27, 454–462 (2021).
Cotugno, N. et al. Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep. 34, 108852 (2021).
Feldstein, L. R. et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325, 1074–1087 (2021).
Weisberg, S. P. et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 22, 25–31 (2021).
Porritt, R. A. et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J. Clin. Investig. 131, e151520 (2021).
Vella, L. A. et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 6, eabf7570 (2021).
Lima, R. et al. Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC Med. Res. Methodol. 20, 228 (2020).
Yonker, L. M. et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Investig. 131, e149633 (2021).
Ouldali, N. et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 325, 855–864 (2021).
Lapp, S. A. et al. Serologic and cytokine signatures in children with multisystem inflammatory syndrome and coronavirus disease 2019. Open Forum Infect. Dis. 9, ofac070 (2022).
Long, Q. X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020).
Porritt, R. A. et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Investig. 131, e146614 (2021).
Natarajan, A. et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Medicine 3, 371–387.e379 (2022).
Ashkenazi-Hoffnung, L. et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr. Infect. Dis. J. 40, e509–e511 (2021).
Borch, L., Holm, M., Knudsen, M., Ellermann-Eriksen, S. & Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur. J. Pediatr. 181, 1597–1607 (2022).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76, e487–e490 (2023).
Bartsch, Y. C. et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci. Transl. Med. 14, eabn9237 (2022).
Levy, M. et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA 327, 281–283 (2022).
Olson, S. M. et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N. Engl. J. Med. 386, 713–723 (2022).
Zambrano, L. D. et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years - United States, July-December 2021. MMWR Morb. Mortal. Wkly Rep. 71, 52–58 (2022).
Wallukat, G. et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 4, 100100 (2021).
Son, K. et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 61, 2200970 (2023).
Muri, J. et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nat. Immunol. 24, 604–611 (2023).
Acknowledgements
We thank the children and their families for participating in this research and Nancy Zimmerman, Mark and Lisa Schwartz, Terry and Susan Ragon, and the SAMANA Kay MGH Research Scholars award for their support.
Funding
We acknowledge support from Massachusetts General Hospital for Children, the Ragon Institute of MGH, MIT, and Harvard, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, and the March of Dimes. We also received support from an anonymous donor (financial support), the NIH (3R37AI080289-11S1, R01AI146785, U19AI42790-01, U19AI135995-02, 1U01CA260476-01, CIVIC75N93019C00052, 5K08HL143183, R01HD100022-02S2) and the Gates Foundation Global Health Vaccine Accelerator Platform funding (OPP1146996 and INV-001650).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data or analysis and interpretation of data: M.D.B., Y.C.B., J.P.D., B.P.B., M.L., J.K., A.S.K., A.G.E., A.F., G.A., L.M.Y. Drafting the article or revising it critically for important intellectual content: M.D.B., Y.C.B., A.S.K., A.G.E., A.F., G.A., L.M.Y. Final approval of the version to be published: M.D.B., Y.C.B., J.P.D., B.P.B., M.L., J.K., A.S.K., A.G.E., A.F., G.A., L.M.Y.
Corresponding author
Ethics declarations
Competing interests
G.A. is a V.P. at Moderna, a founder and equity holder of Seromyx Systems, and an employee and equity holder of Leyden Labs. G.A.’s interests were reviewed and are managed by MGH and Partners HealthCare in accordance with their conflict-of-interest policies. A.G.E. reported serving as a medical advisor for Mirvie, Inc. and receiving research funding from Merck & Co. outside of the scope of the submitted work. No other disclosures were reported. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval and consent to participate
Informed consent, and where appropriate, assent, was verbally obtained from participants and/or parents/guardians involved in the study. The study was approved by the Institutional Review Board of Massachusetts General Brigham (IRB #2020P000955, approved 12/14/20).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burns, M.D., Bartsch, Y.C., Davis, J.P. et al. Long-term humoral signatures following acute pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children. Pediatr Res 94, 1327–1334 (2023). https://doi.org/10.1038/s41390-023-02627-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-023-02627-w