Abstract
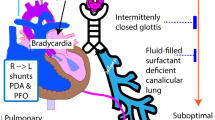
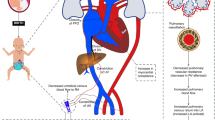
We aimed to review the physiology and evidence behind cardiorespiratory interactions during the transitional circulation of extremely preterm infants with fragile physiology and to propose a framework for future research. Cord clamping strategies have a great impact on initial haemodynamic changes, and appropriate transition can be facilitated by establishing spontaneous ventilation before cord clamping. Mechanical ventilation modifies preterm transitional haemodynamics, with positive pressure ventilation affecting the right and left heart loading conditions. Pulmonary vascular resistances can be minimized by ventilating with optimal lung volumes at functional residual capacity, and other pulmonary vasodilator treatments such as inhaled nitric oxide can be used to improve ventilation/perfusion mismatch. Different cardiovascular drugs can be used to provide support during transition in this population, and it is important to understand both their cardiovascular and respiratory effects, in order to provide adequate support to vulnerable preterm infants and improve outcomes. Current available non-invasive bedside tools, such as near-infrared spectroscopy, targeted neonatal echocardiography, or lung ultrasound offer the opportunity to precisely monitor cardiorespiratory interactions in preterm infants. More research is needed in this field using precision medicine to strengthen the benefits and avoid the harms associated to early neonatal interventions.
Impact
-
In extremely preterm infants, haemodynamic and respiratory transitions are deeply interconnected, and their changes have a key impact in the establishment of lung aireation and postnatal circulation.
-
We describe how mechanical ventilation modifies heart loading conditions and pulmonary vascular resistances in preterm patients, and how hemodynamic interventions such as cord clamping strategies or cardiovascular drugs affect the infant respiratory status.
-
Current available non-invasive bedside tools can help monitor cardiorespiratory interactions in preterm infants. We highlight the areas of research in which precision medicine can help strengthen the benefits and avoid the harms associated to early neonatal interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data from the literature searches performed during the current study are available from the corresponding author on reasonable request.
References
Harrison, M. S. & Goldenberg, R. L. Global burden of prematurity. Semin. Fetal. Neonatal Med. 21, 74–79 (2016).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Shah, P. S. et al. Actuarial Survival Based on Gestational Age in Days at Birth for Infants Born at <26 Weeks of Gestation. J. Pediatr. 225, 97–102.e3 (2020).
Stoll, B. J. et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Stolz, C., et al. Bronchopulmonary dysplasia: temporal trend from 2010 to 2019 in the Brazilian Network on Neonatal Research. Arch. Dis. Child Fetal Neonatal. Ed. 109, 328–335 (2024).
Spencer, B. L. & Mychaliska, G. B. Milestones for clinical translation of the artificial placenta. Semin. Fetal Neonatal Med. 27, 101408 (2022).
De Bie, F. R., Davey, M. G., Larson, A. C., Deprest, J. & Flake, A. W. Artificial placenta and womb technology: Past, current, and future challenges towards clinical translation. Prenat. Diagn. 41, 145–158 (2021).
Sehgal, A., Ruoss, J. L., Stanford, A. H., Lakshminrusimha, S. & McNamara, P. J. Hemodynamic consequences of respiratory interventions in preterm infants. J. Perinatol. 42, 1153–1160 (2022).
Relangi, D. et al. Changes in Patent Ductus Arteriosus Treatment Strategy and Respiratory Outcomes in Premature Infants. J. Pediatr. 235, 58–62 (2021).
Murthy, P. et al. Neuroprotection Care Bundle Implementation to Decrease Acute Brain Injury in Preterm Infants. Pediatr. Neurol. 110, 42–48 (2020).
Molloy E. J., et al. Neuroprotective therapies in the NICU in preterm infants: present and future (Neonatal Neurocritical Care Series). Pediatr. Res. 95, 1224–1236 (2024).
Pavlek, L. R. et al. Eligibility Criteria and Representativeness of Randomized Clinical Trials That Include Infants Born Extremely Premature: A Systematic Review. J. Pediatr. 235, 63–74.e12 (2021).
Janvier, A. & Farlow, B. The ethics of neonatal research: An ethicist’s and a parents’ perspective. Semin. Fetal Neonatal Med. 20, 436–441 (2015). Dec.
Smith, A. & El-Khuffash, A. Patent Ductus Arteriosus Clinical Trials: Lessons Learned and Future Directions. Child 8, 47 (2021). Jan 15.
Keszler, M. Volume-targeted ventilation: one size does not fit all. Evidence-based recommendations for successful use. Arch. Dis. Child Fetal Neonatal Ed. 104, F108–F112 (2019).
Kumar, N., Akangire, G., Sullivan, B., Fairchild, K. & Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatr. Res. 87, 210–220 (2020).
Baethge, C., Goldbeck-Wood, S. & Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 4, 5 (2019).
Chakkarapani, A. A., et al. Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr Res. https://doi.org/10.1038/s41390-022-02427-8 (2023).
te Pas, A. B., Davis, P. G., Hooper, S. B. & Morley, C. J. From liquid to air: breathing after birth. J. Pediatr. 152, 607–611 (2008).
Hooper, S. B., Polglase, G. R. & Roehr, C. C. Cardiopulmonary changes with aeration of the newborn lung. Paediatr. Respir. Rev. 16, 147–150 (2015).
Shekerdemian, L. & Bohn, D. Cardiovascular effects of mechanical ventilation. Arch. Dis. Child 80, 475–480 (1999).
Bensley, J. G., Moore, L., De Matteo, R., Harding, R. & Black, M. J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr. Res. 83, 880–888 (2018).
Hooper, S. B. et al. Cardiovascular transition at birth: a physiological sequence. Pediatr. Res. 77, 608–614 (2015).
Chandrasekharan, P. et al. Effect of various inspired oxygen concentrations on pulmonary and systemic hemodynamics and oxygenation during resuscitation in a transitioning preterm model. Pediatr. Res. 84, 743–750 (2018).
Bhatt, S., Polglase, G. R., Wallace, E. M., Te Pas, A. B. & Hooper, S. B. Ventilation before Umbilical Cord Clamping Improves the Physiological Transition at Birth. Front. Pediatr. 2, 113 (2014).
Seidler, A. L. et al. Deferred cord clamping, cord milking, and immediate cord clamping at preterm birth: a systematic review and individual participant data meta-analysis. Lancet 402, 2209–2222 (2023).
McAdams, R. M., Fay, E. & Delaney, S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J. Perinatol. 38, 245–250 (2018).
Katheria, A., Reister, F. & Essers, J. et al. Association of Umbilical Cord Milking vs Delayed Umbilical Cord Clamping With Death or Severe Intraventricular Hemorrhage Among Preterm Infants. JAMA 322, 1877–1886 (2019).
Katheria, A. et al. Umbilical Cord Milking Versus Delayed Cord Clamping in Infants 28 to 32 Weeks: A Randomized Trial. Pediatrics 152, e2023063113 (2023).
Raina, J. S. et al. Resuscitation with Intact Cord Versus Clamped Cord in Late Preterm and Term Neonates: A Randomized Controlled Trial. J. Pediatr. 254, 54–60.e4 (2023).
Katheria, A. C. Neonatal Resuscitation with an Intact Cord: Current and Ongoing Trials. Child. 6, 60 (2019).
Subramaniam, P., Ho, J. J. & Davis, P. G. Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants. Cochrane Database Syst. Rev. 10, CD001243 (2021).
Ramaswamy, V. V., More, K., Roehr, C. C., Bandiya, P. & Nangia, S. Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: Systematic review and network meta-analysis. Pediatr. Pulmonol. 55, 2940–2963 (2020).
Moritz, B., Fritz, M., Mann, C. & Simma, B. Nasal continuous positive airway pressure (n-CPAP) does not change cardiac output in preterm infants. Am. J. Perinatol. 25, 105–109 (2008).
Kuypers, K. L. A. M. et al. Exerted force on the face mask in preterm infants at birth is associated with apnoea and bradycardia. Resuscitation 194, 110086 (2024).
Polglase, G. R. et al. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J. Appl Physiol. 99, 1453–1461 (2005).
Zannin, E. et al. Relationship between Mean Airways Pressure, Lung Mechanics, and Right Ventricular Output during High-Frequency Oscillatory Ventilation in Infants. J. Pediatr. 180, 110–115 (2017).
Osborn, D. A., Evans, N. & Kluckow, M. Left ventricular contractility in extremely premature infants in the first day and response to inotropes. Pediatr. Res. 61, 335–340 (2007).
Simmons, D. H., Linde, L. M., Miller, J. H. & O’Reilly, R. J. Relation between lung volume and pulmonary vascular resistance. Circ. Res. 9, 465–471 (1961).
Tana, M. et al. Determination of Lung Volume and Hemodynamic Changes During High-Frequency Ventilation Recruitment in Preterm Neonates With Respiratory Distress Syndrome. Crit. Care Med. 43, 1685–1691 (2015).
Klingenberg, C., Wheeler, K. I., McCallion, N., Morley, C. J. & Davis, P. G. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst. Rev. 10, CD003666, (2017).
Bugiera, M., Szczapa, T., Sowińska, A., Roehr, C. C. & Szymankiewicz-Bręborowicz, M. Cerebral oxygenation and circulatory parameters during pressure-controlled vs volume-targeted mechanical ventilation in extremely preterm infants. Adv. Clin. Exp. Med. 29, 1325–1329 (2020).
Cools, F., Offringa, M. & Askie, L. M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst. Rev. 2015, CD000104 (2015).
Simma, B., Fritz, M., Fink, C. & Hammerer, I. Conventional ventilation versus high-frequency oscillation: hemodynamic effects in newborn babies. Crit. Care Med. 28, 227–231 (2000).
Cambonie, G. et al. Haemodynamic features during high-frequency oscillatory ventilation in preterms. Acta Paediatr. 92, 1068–1073 (2003).
Ayoub, D., Elmashad, A., Rowisha, M., Eltomey, M. & El Amrousy, D. Hemodynamic effects of high-frequency oscillatory ventilation in preterm neonates with respiratory distress syndrome. Pediatr. Pulmonol. 56, 424–432 (2021).
Elgin, T. G., Stanford, A. H. & Klein, J. M. First intention high-frequency jet ventilation for periviable infants. Curr. Opin. Pediatr. 34, 165–169 (2022).
Hibberd, J., et al. Neonatal high-frequency oscillatory ventilation: where are we now? Arch. Dis. Child Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2023-325657 (2023).
Ali, S. K., Stanford, A. H., McNamara, P. J. & Gupta, S. Surfactant and neonatal hemodynamics during the postnatal transition. Semin. Fetal Neonatal Med. 28, 101498 (2023).
Katheria, A. C. & Leone, T. A. Changes in hemodynamics after rescue surfactant administration. J. Perinatol. 33, 525–528 (2013).
Sehgal, A. et al. Haemodynamic changes after delivery room surfactant administration to very low birth weight infants. Arch. Dis. Child Fetal Neonatal Ed. 95, F345–F351 (2010).
Vitali, F. et al. Pilot observational study on haemodynamic changes after surfactant administration in preterm newborns with respiratory distress syndrome. Ital. J. Pediatr. 40, 26, (2014).
Mielgo, V. et al. Structural and haemodynamic evaluation of less invasive surfactant administration during nasal intermittent positive pressure ventilation in surfactant-deficient newborn piglets. PLoS One 18, e0284750 (2023).
Rey-Santano, C. et al. Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr. Res. 73, 639–646 (2013).
Hanke, K. et al. The effect of less invasive surfactant administration on cerebral oxygenation in preterm infants. Acta Paediatr. 109, 291–299 (2020).
Ichinose, F., Roberts, J. D. Jr & Zapol, W. M. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation 109, 3106–3111 (2004).
Giesinger, R. E. & McNamara, P. J. Hemodynamic instability in the critically ill neonate: An approach to cardiovascular support based on disease pathophysiology. Semin. Perinatol. 40, 174–188 (2016).
Chandrasekharan, P., Lakshminrusimha, S. & Abman, S. H. When to say no to inhaled nitric oxide in neonates? Semin. Fetal Neonatal Med. 26, 101200 (2021).
Al-Ghanem, G. et al. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J. Perinatol. 37, 414–419 (2017).
Mirza, H., Mandell, E. W., Kinsella, J. P., McNamara, P. J. & Abman, S. H. Pulmonary Vascular Phenotypes of Prematurity: The Path to Precision Medicine. J. Pediatr. 259, 113444 (2023).
Zhu, F. et al. Cardiopulmonary physiological effects of diuretic therapy in preterm infants with chronic pulmonary hypertension. J. Perinatol. 43, 1288–1294 (2023).
Burns, M. L. et al. Inotropic Therapy in Newborns, A Population-Based National Registry Study. Pediatr. Crit. Care Med. 17, 948–956 (2016).
Bravo, M. C. et al. Validity of Biomarkers of Early Circulatory Impairment to Predict Outcome: A Retrospective Analysis. Front Pediatr. 7, 212 (2019).
Logan, J. W. et al. Early postnatal hypotension and developmental delay at 24 months of age among extremely low gestational age newborns. Arch. Dis. Child Fetal Neonatal Ed. 96, F321–F328 (2011).
Dempsey, E. M. & Barrington, K. J. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J. Perinatol. 27, 469–478 (2007).
Aldana-Aguirre, J. C., Deshpande, P., Jain, A. & Weisz, D. E. Physiology of Low Blood Pressure During the First Day After Birth Among Extremely Preterm Neonates. J. Pediatr. 236, 40–46.e3 (2021).
Keir A. K. et al. International, multicentre, observational study of fluid bolus therapy in neonates. J. Paediatr. Child Health. 55, 632–639 (2019).
Kluckow, M. & Evans, N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J. Pediatr. 129, 506–512 (1996).
Sehgal, A. & Gauli, B. Changes in respiratory mechanics in response to crystalloid infusions in extremely premature infants. Am. J. Physiol. Lung Cell Mol. Physiol. 325, L819–L825 (2023).
Osborn, D., Evans, N. & Kluckow, M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J. Pediatr. 140, 183–191 (2002).
Bravo, M. C. et al. Randomized, Placebo-Controlled Trial of Dobutamine for Low Superior Vena Cava Flow in Infants. J. Pediatr. 167, 572–8.e1-2 (2015).
Al-Salam, Z., Johnson, S., Abozaid, S., Bigam, D. & Cheung, P. Y. The hemodynamic effects of dobutamine during reoxygenation after hypoxia: a dose-response study in newborn pigs. Shock 28, 317–325 (2007).
Pellicer, A. et al. Pharmacokinetic study (phase I-II) of a new dobutamine formulation in preterm infants immediately after birth. Pediatr. Res. 89, 981–986 (2021).
Noori, S. & Seri, I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin. Perinatol. 39, 221–238 (2012).
Pellicer, A. et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 115, 1501–1512 (2005).
Liet, J. M. et al. Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J. Pediatr. 140, 373–375 (2002).
Bouissou, A. et al. Hypotension in preterm infants with significant patent ductus arteriosus: effects of dopamine. J. Pediatr. 153, 790–794 (2008). Dec.
McNamara, P. J., Giesinger, R. E. & Lakshminrusimha, S. Dopamine and Neonatal Pulmonary Hypertension-Pressing Need for a Better Pressor? J. Pediatr. 246, 242–250 (2022).
Valverde, E. et al. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: analysis of systemic effects and neonatal clinical outcomes. Pediatrics 117, e1213–e1222 (2006).
Baske, K., Saini, S. S., Dutta, S. & Sundaram, V. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur. J. Pediatr. 177, 1335–1342 (2018).
Cheung, P. Y. & Barrington, K. J. The effects of dopamine and epinephrine on hemodynamics and oxygen metabolism in hypoxic anesthetized piglets. Crit. Care 5, 158–166 (2001).
Rowcliff, K., de Waal, K., Mohamed, A. L. & Chaudhari, T. Noradrenaline in preterm infants with cardiovascular compromise. Eur. J. Pediatr. 175, 1967–1973 (2016).
Gupta, S., Agrawal, G., Thakur, S., Gupta, A. & Wazir, S. The effect of norepinephrine on clinical and hemodynamic parameters in neonates with shock: a retrospective cohort study. Eur. J. Pediatr. 181, 2379–2387 (2022).
Lu, P., Sun, Y., Gong, X., Li, Z. & Hong, W. Use of norepinephrine in preterm neonates with dopamine-resistant shock: a retrospective single-centre cross-sectional study. BMJ Paediatr. Open 7, e001804 (2023).
Tourneux, P., Rakza, T., Bouissou, A., Krim, G. & Storme, L. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J. Pediatr. 153, 345–349 (2008).
Barrett, L. K., Singer, M. & Clapp, L. H. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit. Care Med. 35, 33–40 (2007).
Rios, D. R. & Kaiser, J. R. Vasopressin versus dopamine for treatment of hypotension in extremely low birth weight infants: a randomized, blinded pilot study. J. Pediatr. 166, 850–855 (2015).
Mohamed, A., Nasef, N., Shah, V. & McNamara, P. J. Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr. Crit. Care Med. 15, 148–154 (2014).
McNamara, P. J., Shivananda, S. P., Sahni, M., Freeman, D. & Taddio, A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr. Crit. Care Med. 14, 74–84 (2013).
Khazin, V. et al. Milrinone and nitric oxide: combined effect on pulmonary artery pressures after cardiopulmonary bypass in children. J. Cardiothorac. Vasc. Anesth. 18, 156–159 (2004).
Matsushita, F. Y., et al. Reassessing the role of milrinone in the treatment of heart failure and pulmonary hypertension in neonates and children: a systematic review and meta-analysis. Eur. J. Pediatr. 183, 543–555 (2024).
James, A. T. et al. Treatment of premature infants with pulmonary hypertension and right ventricular dysfunction with milrinone: a case series. J. Perinatol. 35, 268–273 (2015).
McNamara, P. J., Abman, S., Levy, P. T. Reengagement with Physiology in Neonatal Heart and Lung Care: A Priority for Training and Practice. J. Pediatr. 268, 113947 (2024).
Bravo, M. C., Jiménez, R., Parrado-Hernández, E., Fernández, J. J., Pellicer, A. Predicting the effectiveness of drugs used for treating cardiovascular conditions in newborn infants. Pediatr. Res. 95, 1124–1131 (2024).
Dempsey, E. M. What Should We Do about Low Blood Pressure in Preterm Infants. Neonatology 111, 402–407 (2017).
Mullaly, R., El-Khuffash, A. F. Haemodynamic assessment and management of hypotension in the preterm. Arch. Dis. Child Fetal Neonatal Ed. 109, 120–127 (2024).
Vesoulis, Z., Tims, A., Lodhi, H., Lalos, N. & Whitehead, H. Racial discrepancy in pulse oximeter accuracy in preterm infants. J. Perinatol. 42, 79–85 (2022).
Pellicer, A. & Bravo, M. C. Near-infrared spectroscopy: a methodology-focused review. Semin. Fetal Neonatal Med. 16, 42–49 (2011).
Martini, S. et al. Cardiovascular and cerebrovascular responses to cardio-respiratory events in preterm infants during the transitional period. J. Physiol. 598, 4107–4119 (2020).
Pellicer, A. et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 104, 171–178 (2013).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635 (2015).
Hansen, M. L. et al. Cerebral Oximetry Monitoring in Extremely Preterm Infants. N. Engl. J. Med. 388, 1501–1511 (2023).
Pichler, G. et al. Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): multicentre randomised phase 3 clinical trial. BMJ 380, e072313 (2023).
McNamara, P. J. et al. Guidelines and Recommendations for Targeted Neonatal Echocardiography and Cardiac Point-of-Care Ultrasound in the Neonatal Intensive Care Unit: An Update from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 37, 171–215 (2024).
Bischoff, A. R. et al. Targeted Neonatal Echocardiography in Patients With Hemodynamic Instability. Pediatrics 150, e2022056415I (2022).
Giesinger, R. E., Hobson, A. A., Bischoff, A. R., Klein, J. M. & McNamara, P. J. Impact of early screening echocardiography and targeted PDA treatment on neonatal outcomes in “22-23” week and “24-26” infants. Semin Perinatol. 47, 151721 (2023).
Sankaran, D. et al. Non-invasive carbon dioxide monitoring in neonates: methods, benefits, and pitfalls. J. Perinatol. 41, 2580–2589 (2021).
Jain, D. & D’Ugard, C. et al. Use of a Mechanical Ventilator with Respiratory Function Monitoring Provides More Consistent Ventilation during Simulated Neonatal Resuscitation. Neonatology 117, 151–158 (2020).
Raimondi, F., Yousef, N., Migliaro, F., Capasso, L. & De Luca, D. Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr. Res. 90, 524–531 (2021).
Raimondi, F., et al. External Validation of a Multivariate Model for Targeted Surfactant Replacement. Neonatology 121, 17–24 (2024).
Mohsen, N., Solis-Garcia, G., Jasani, B., Nasef, N. & Mohamed, A. Accuracy of lung ultrasound in predicting extubation failure in neonates: A systematic review and meta-analysis. Pediatr. Pulmonol. 58, 2846–2856 (2023).
Rodriguez-Fanjul, J. et al. Lung ultrasound to evaluate lung recruitment in neonates with respiratory distress (RELUS study). Pediatr. Pulmonol. 57, 2502–2510 (2022).
Buonsenso, D. et al. Lung ultrasound to detect cardiopulmonary interactions in acutely ill children. Pediatr. Pulmonol. 57, 483–497 (2022).
Onland, W. et al. Precision Medicine in Neonates: Future Perspectives for the Lung. Front. Pediatr. 8, 586061 (2020).
Funding
This article did not receive any specific financial assistance. G.S.G. is funded by the program Rio Hortega, co-funded by the Spanish Ministry of Science and the European Union.
Author information
Authors and Affiliations
Contributions
Gonzalo Solís García, María Carmen Bravo and Adelina Pellicer conceptualized and designed the project. Gonzalo Solís García performed the literature search and wrote the initial draft of the manuscript. María Carmen Bravo and Adelina Pellicer critically reviewed and edited the original manuscript. All three authors have approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solís-García, G., Bravo, M.C. & Pellicer, A. Cardiorespiratory interactions during the transitional period in extremely preterm infants: a narrative review. Pediatr Res 97, 871–879 (2025). https://doi.org/10.1038/s41390-024-03451-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03451-6
This article is cited by
-
Association between early-phase opioid use and outcomes in extremely preterm infants: A nationwide study
Pediatric Research (2025)
-
Enhancing neonatal resuscitation outcomes: bridging theory and practice
European Journal of Pediatrics (2025)