Abstract
Objectives
Early exposure to mother’s own milk (MOM) promotes intestinal barrier maturation in preterm infants. We hypothesized (1) donor human milk (DHM) supplementation reduces intestinal permeability (IP) similar to exclusive MOM and (2) early HM exposure and low IP at 7–10 days postnatal age (PNA) are associated with improved growth outcomes.
Methods
IP was measured by the standard sugar absorption test (SAT) in infants <33 weeks gestation between 7–10 days PNA. Nutritional and anthropometric data were recorded. Postnatal growth failure (PNGF) was defined as a decrease in weight z-score >1 from birth to discharge to home.
Results
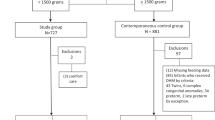
Of 158 preterm infants, the mean (SD) gestational age was 29.9(2.3) weeks and birthweight 1388(424) g. Diet prior to SAT was exclusive MOM [N = 55(35%)], DHM ± MOM [N = 52(33%)], or preterm formula±MOM [N = 51(32%)]. The mean Lactulose(La)/Rhamnose(Rh) ratio was lower in the exclusive MOM [0.06(0.07)] and DBM ± MOM [0.05(0.07)] groups compared to the preterm formula±MOM group [0.11(0.11)], p < 0.01). Cumulative intake >150 ml/kg MOM ± DHM, but not preterm formula within 7–10 days PNA was associated with early intestinal barrier maturation. Low IP was not associated with lower risk of PNGF at discharge.
Conclusions
Low IP is associated with cumulative intake of MOM alone or supplemented with DHM > 150 ml/kg within 7–10 days PNA.
Clinical trial registration
Clinicaltrials.gov NCT01756040; web link to study on registry:https://clinicaltrials.gov/study/NCT01756040.
Impact
-
Key message Early intestinal barrier maturation is associated with cumulative intake of exclusive MOM alone or supplemented with DHM > 150 ml/kg within 7–10 days after birth, but is not associated with lower risk of PNGF at time of discharge.
-
What it adds to existing literature? This observational study is the first study to demonstrate that supplemental DHM promotes intestinal barrier maturation similar to MOM alone.
-
What is the impact? The findings underscore the importance of early introduction of human milk feeds as MOM or MOM supplemented with DHM in sufficient volume to promote early intestinal barrier maturation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Deidentified individual participant data (including data dictionaries) will be made available, in addition to the study protocol, the statistical analysis plan and the informed consent form. The data will be made available upon publication to researchers who provide a methodically sound proposal. Requests should be submitted to ssundararajan@som.umaryland.edu.
References
Halpern, M. D. & Denning, P. W. The role of intestinal epithelial barrier function in the development of Nec. Tissue Barriers 3, e1000707 (2015).
Beach, R. C., Menzies, I. S., Clayden, G. S. & Scopes, J. W. Gastrointestinal permeability changes in the preterm neonate. Arch. Dis. Child 57, 141–145 (1982).
Corpeleijn, W. E., van Elburg, R. M., Kema, I. P. & van Goudoever, J. B. Assessment of intestinal permeability in (premature) neonates by sugar absorption tests. Methods Mol. Biol. 763, 95–104 (2011).
Stratiki, Z. et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 83, 575–579 (2007).
van Elburg, R. M., Fetter, W. P., Bunkers, C. M. & Heymans, H. S. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch. Dis. Child Fetal Neonatal Ed. 88, F52–F55 (2003).
Taylor, S. N., Basile, L. A., Ebeling, M. & Wagner, C. L. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed. Med 4, 11–15 (2009).
Saleem, B. et al. Intestinal barrier maturation in very low birthweight infants: relationship to feeding and antibiotic exposure. J. Pediatr. 183, 31–36.e31 (2017).
Ma, B. et al. Microbial biomarkers of intestinal barrier maturation in preterm infants. Front Microbiol 9, 2755 (2018).
Ma, B. et al. Highly specialized carbohydrate metabolism capability in bifidobacterium strains associated with intestinal barrier maturation in early preterm infants. mBio 13, e0129922 (2022).
Lemme-Dumit, J. M. et al. Altered gut microbiome and fecal immune phenotype in early preterm infants with leaky gut. Front Immunol. 13, 815046 (2022).
Shulman, R. J. et al. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr. Res 44, 519–523 (1998).
Sullivan, S. et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 156, 562–567.e561 (2010).
Hair, A. B. et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed. Med 11, 70–74 (2016).
Hair, A. B. et al. Neurodevelopmental outcomes of extremely preterm infants fed an exclusive human milk-based diet versus a mixed human milk + bovine milk-based diet: a multi-center study. J. Perinatol. 42, 1485–1488 (2022).
Section on, B. Breastfeeding and the use of human milk. Pediatrics 129, e827-e841 (2012).
Stefanescu, B. M., Camacho, J., Stefanescu, A. R., DuPont, T. & Leung, R. Impact of a standardized nutrition bundle including donor human milk on hospital outcomes in very low birth-weight (VLBW) infants in a safety-net hospital. Adv. Neonatal Care 22, 503–512 (2022).
Ramirez, C. B. et al. Effects of Human Milk On Body Composition And Growth In Very Low Birthweight Infants. Pediatr. Res 93, 2028–2035 (2023).
Cartagena, D. et al. Differences in neonatal outcomes among premature infants exposed to mother’s own milk versus donor human milk. Adv. Neonatal Care 22, 539–549 (2022).
Sanchez-Rosado, M. et al. Growth after implementing a donor breast milk program in neonates <33 weeks gestational age or birthweight <1500 grams: retrospective cohort study. J. Perinatol. 43, 608–615 (2023).
Perrin, M. T. et al. The nutritional composition and energy content of donor human milk: a systematic review. Adv. Nutr. 11, 960–970 (2020).
Marx, C. et al. Human milk oligosaccharide composition differs between donor milk and mother’s own milk in the NICU. J. Hum. Lact 30, 54–61 (2014).
John, A. et al. Macronutrient variability in human milk from donors to a milk bank: implications for feeding preterm infants. PLoS One 14, e0210610 (2019).
Parra-Llorca, A. et al. Preterm gut microbiome depending on feeding type: significance of donor human milk. Front Microbiol 9, 1376 (2018).
Brownell, E. A. et al. Dose-response relationship between donor human milk, mother’s own milk, preterm formula, and neonatal growth outcomes. J. Pediatr. Gastroenterol. Nutr. 67, 90–96 (2018).
Colaizy, T. T. Effects of milk banking procedures on nutritional and bioactive components of donor human milk. Semin Perinatol. 45, 151382 (2021).
Chou, J. H., Roumiantsev, S. & Singh, R. Peditools electronic growth chart calculators: applications in clinical care, research, and quality improvement. J. Med Internet Res 22, e16204 (2020).
Ofek Shlomai, N. et al. Neonatal morbidities and postnatal growth failure in very low birth weight, very preterm infants. Acta Paediatr. 111, 1536–1545 (2022).
Bagga, N. et al. Extrauterine growth restriction: need for an accurate definition. Newborn (Clarksville) 2, 198–202 (2023).
Peila, C. et al. Extrauterine growth restriction: definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients 12, 49–60 (2020).
Patel, A. L., Engstrom, J. L., Meier, P. P. & Kimura, R. E. Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics 116, 1466–1473 (2005).
Patel, A. L., Engstrom, J. L., Meier, P. P., Jegier, B. J. & Kimura, R. E. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J. Perinatol. 29, 618–622 (2009).
Fenton, T. R. et al. Accuracy of preterm infant weight gain velocity calculations vary depending on method used and infant age at time of measurement. Pediatr. Res 85, 650–654 (2019).
Berman, L. & Moss, R. L. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med 16, 145–150 (2011).
Fatemizadeh, R. et al. Incidence of spontaneous intestinal perforations exceeds necrotizing enterocolitis in extremely low birth weight infants fed an exclusive human milk-based diet: a single center experience. J. Pediatr. Surg. 56, 1051–1056 (2021).
Buckle, A. & Taylor, C. Cost and cost-effectiveness of donor human milk to prevent necrotizing enterocolitis: systematic review. Breastfeed. Med 12, 528–536 (2017).
Bajwa, R. U. et al. Infant nutrition (donor human milk vs. maternal milk) and long-term neurodevelopmental and growth outcomes in very low birth weight infants. J. Matern Fetal Neonatal Med 35, 10025–10029 (2022).
Noel, G. et al. Human breast milk enhances intestinal mucosal barrier function and innate immunity in a healthy pediatric human enteroid model. Front Cell Dev. Biol. 9, 685171 (2021).
Carneiro, L. et al. The sterilization of human milk by holder pasteurization or by high hydrostatic pressure processing leads to differential intestinal effects in mice. Nutrients 15, 4043 (2023).
Rochow, N. et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr. Res 79, 870–879 (2016).
Fenton, T. R. et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J. Perinatol. 40, 704–714 (2020).
El Rafei, R. et al. Postnatal growth restriction and neurodevelopment at 5 years of age: a European extremely preterm birth cohort study. Arch. Dis. Child Fetal Neonatal Ed. 108, 492–498 (2023).
Lunn, P. G., Northrop-Clewes, C. A. & Downes, R. M. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338, 907–910 (1991).
Campbell, R. K. et al. Environmental enteric dysfunction and systemic inflammation predict reduced weight but not length gain in rural Bangladeshi children. Br. J. Nutr. 119, 407–414 (2018).
Stevens, T. P. et al. Statewide initiative to reduce postnatal growth restriction among infants <31 weeks of gestation. J. Pediatr. 197, 82–89.e82 (2018).
Acknowledgements
We thank Dr. Jonathan Meddings, University of Calgary, Calgary, Alberta, Canada for the HPLC analysis of urine samples and Elise Janofsky for research assistance. This study was supported in part by The Gerber Foundation (award identifier 6361), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (R21DK123674), and a University of Maryland Institute for Clinical and Translational Research Accelerated Translational Incubator Pilot award.
Author information
Authors and Affiliations
Contributions
Drs. Sripriya Sundararajan, Rose M. Viscardi, and Bing Ma conceptualized and designed the study, obtained funding, designed the data collection instruments, coordinated and supervised data collection, participated in data analysis and interpretation, drafted the initial manuscript, and critically reviewed and revised the manuscript. Dr Lisa Roskes coordinated and supervised data collection, participated in data analysis and interpretation, contributed to drafting the initial manuscript and critically reviewed and revised the manuscript for important intellectual content. Dr Mathangi Gopalakrishnan, and Athanasios Chamzas, reviewed the collected data, carried out the statistical analyses, and critically reviewed and revised the manuscript. Dr. Alex Medina contributed to statistical analysis and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Consent statement
Patient consent was obtained as per institutional guidelines prior to the enrollment of the study subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roskes, L., Chamzas, A., Ma, B. et al. Early human milk feeding: Relationship to intestinal barrier maturation and postnatal growth. Pediatr Res 97, 2065–2073 (2025). https://doi.org/10.1038/s41390-024-03622-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03622-5