Abstract
Background
Persistent pulmonary hypertension of the newborn (PPHN) affects systemic oxygenation and may worsen brain injury in infants with neonatal encephalopathy (NE). Evidence suggests that higher cerebral regional oxygenation (crSO2) indicates derangement in cerebral autoregulation, energy metabolism, and blood flow following NE. Our aim was to evaluate the impact of PPHN on crSO2, in infants with NE treated with therapeutic hypothermia (TH).
Methods
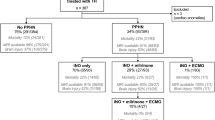
We retrospectively evaluated infants with NE and PPHN vs without PPHN, between 2018-2022. Linear regression analysis was performed to evaluate the impact of PPHN on crSO2 and total MRI score, adjusted for perinatal factors.
Results
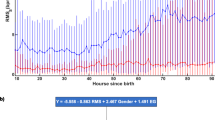
164 infants were analyzed, including 19(12%) with PPHN and 145(88%) without. PPHN-infants had significantly higher crSO2 during rewarming and post-rewarming compared to non-PPHN infants (87 ± 6 vs 80 ± 6, p = 0.001; 87 ± 5 vs 80 ± 7, p = 0.008, respectively), and a significantly higher total MRI score [7(2–19) vs 1(0–3), p < 0.001]. PPHN was significantly associated with higher crSO2 during rewarming (b = 6.21, 95% CI 2.37–10.04, p = 0.002) and post-rewarming (b = 8.60, 95% CI 2.28–14.91, p = 0.009), and total MRI score (b = 7.42, 95% CI 4.88–9.95, p < 0.001).
Conclusions
PPHN was associated with higher crSO2 during and after rewarming, and worse brain MRI score, indicating a significant impact of PPHN on brain injury in infants with NE undergoing TH.
Impact
-
Cerebral oxygenation was significantly higher in infants with neonatal encephalopathy (NE) and persistent pulmonary hypertension (PPHN) compared to infants without PPHN, during the rewarming and post-rewarming periods of therapeutic hypothermia (TH).
-
PPHN is associated with brain injury in infants with NE undergoing TH.
-
In infants with NE and PPHN, monitoring of cerebral oxygenation would help detect infants at higher risk of adverse outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Azzopardi, D. et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr. Res. 25, 445–451 (1989).
Hassell, K. J., Ezzati, M., Alonso-Alconada, D., Hausenloy, D. J. & Robertson, N. J. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch. Dis. Child. Fetal Neonatal Ed. 100, F541–F552 (2015).
Meek, J. H. et al. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch. Dis. Child. Fetal Neonatal Ed. 81, F110–F115 (1999).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, CD003311 (2013).
Lakshminrusimha, S. et al. Pulmonary Hypertension Associated with Hypoxic-Ischemic Encephalopathy-Antecedent Characteristics and Comorbidities. J. Pediatr. 196, 45–51 e43 (2018).
Shankaran, S. et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics 122, e791–e798 (2008).
Garvey, A. A. & Dempsey, E. M. Applications of near infrared spectroscopy in the neonate. Curr. Opin. Pediatr. 30, 209–215 (2018).
Sood, B. G., McLaughlin, K. & Cortez, J. Near-infrared spectroscopy: applications in neonates. Semin. Fetal Neonatal Med. 20, 164–172 (2015).
Toet, M. C., Lemmers, P. M. A., van Schelven, L. J. & van Bel, F. Cerebral Oxygenation and Electrical Activity After Birth Asphyxia: Their Relation to Outcome. Pediatrics 117, 333–339 (2006).
Arriaga-Redondo, M. et al. Lack of Variability in Cerebral Oximetry Tendency in Infants with Severe Hypoxic-Ischemic Encephalopathy Under Hypothermia. Ther. Hypothermia Temp. Manag 9, 243–250 (2019).
Ancora, G. et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 35, 26–31 (2013).
Gagnon, M.-H. & Wintermark, P. Effect of persistent pulmonary hypertension on brain oxygenation in asphyxiated term newborns treated with hypothermia. J. Matern. Fetal Neonatal Med. 29, 2049–2055 (2015).
Jain, S. V. et al. Cerebral regional oxygen saturation trends in infants with hypoxic-ischemic encephalopathy. Early Hum. Dev. 113, 55–61 (2017).
Lemmers, P. M. A. et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr. Res. 74, 180–185 (2013).
Nakamura, S. et al. Simultaneous measurement of cerebral hemoglobin oxygen saturation and blood volume in asphyxiated neonates by near-infrared time-resolved spectroscopy. Brain Dev. 37, 925–932 (2015).
Meiners, L. C., Sival, D. A., Bos, A. F., Niezen, C. K. & ter Horst, H. J. Amplitude-Integrated EEG and Cerebral Near-Infrared Spectroscopy in Cooled, Asphyxiated Infants. Am. J. Perinatol. 35, 904–910 (2018).
Peng, S. et al. Does near-infrared spectroscopy identify asphyxiated newborns at risk of developing brain injury during hypothermia treatment? Am. J. Perinatol. 32, 555–564 (2015).
Toet, M. C. & Lemmers, P. M. Brain monitoring in neonates. Early Hum. Dev. 85, 77–84 (2009).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Walsh, B. H. et al. Differences in standardized neonatal encephalopathy exam criteria may impact therapeutic hypothermia eligibility. Pediatr. Res. 92, 791–798 (2022).
More, K., Soni, R., Gupta, S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin. Fetal Neonatal Med. 27, 101366 (2022).
Szakmar, E. et al. Association between cerebral oxygen saturation and brain injury in neonates receiving therapeutic hypothermia for neonatal encephalopathy. J. Perinatol. 41, 269–277 (2021).
Weeke, L. C. et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J. Pediatrics 192, 33–40.e32 (2018).
Cady, E. B. et al. Early cerebral-metabolite quantification in perinatal hypoxic-ischaemic encephalopathy by proton and phosphorus magnetic resonance spectroscopy. Magn. Reson Imaging 15, 605–611 (1997).
Laptook, A. R., Corbett, R. J., Sterett, R., Garcia, D. & Tollefsbol, G. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr. Res. 38, 919–925 (1995).
Tekes, A. et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. AJNR. Am. J. Neuroradiol. 36, 188–193 (2015).
Giesinger, R. E. et al. Impaired Right Ventricular Performance Is Associated with Adverse Outcome after Hypoxic Ischemic Encephalopathy. Am. J. Respirat. Crit. Care Med. 200, 1294–1305 (2019).
Miller, S. et al. Cardiovascular Associations with Abnormal Brain Magnetic Resonance Imaging in Neonates with Hypoxic Ischemic Encephalopathy Undergoing Therapeutic Hypothermia and Rewarming. Am. J. Perinatol. 35, 979–989 (2018).
Al Balushi, A. et al. Hypotension and Brain Injury in Asphyxiated Newborns Treated with Hypothermia. Am. J. Perinatol. 35, 31–38 (2018).
Mohammad, K. et al. Hemodynamic instability associated with increased risk of death or brain injury in neonates with hypoxic ischemic encephalopathy. J. Neonatal Perinat. Med. 10, 363–370 (2017).
Wintermark, P., Hansen, A., Warfield, S. K., Dukhovny, D. & Soul, J. S. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage 85, 287–293 (2014).
Wintermark, P. et al. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am. J. Neuroradiol. 32, 2023–2029 (2011).
Variane, G. F. T., Chock, V. Y., Netto, A., Pietrobom, R. F. R. & Van Meurs, K. P. Simultaneous Near-Infrared Spectroscopy (NIRS) and Amplitude-Integrated Electroencephalography (aEEG): Dual Use of Brain Monitoring Techniques Improves Our Understanding of Physiology. Front. Pediatr. 7, 560 (2019).
Vesoulis, Z. A., Liao, S. M. & Mathur, A. M. Late failure of cerebral autoregulation in hypoxic-ischemic encephalopathy is associated with brain injury: a pilot study. Physiol. Meas. 39, 125004 (2018).
Wu, T. W., Tamrazi, B., Soleymani, S., Seri, I. & Noori, S. Hemodynamic Changes During Rewarming Phase of Whole-Body Hypothermia Therapy in Neonates with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 197, 68–74.e62 (2018).
Gebauer, C. M., Knuepfer, M., Robel-Tillig, E., Pulzer, F. & Vogtmann, C. Hemodynamics among neonates with hypoxic-ischemic encephalopathy during whole-body hypothermia and passive rewarming. Pediatrics 117, 843–850 (2006).
Bacher, A. Effects of body temperature on blood gases. Intensive Care Med. 31, 24–27 (2005).
Chalak, L. F., Tarumi, T. & Zhang, R. The “neurovascular unit approach” to evaluate mechanisms of dysfunctional autoregulation in asphyxiated newborns in the era of hypothermia therapy. Early Hum. Dev. 90, 687–694 (2014).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatr. 117, 119–125 (1990).
Wayock, C. P. et al. Perinatal risk factors for severe injury in neonates treated with whole-body hypothermia for encephalopathy. Am. J. Obstet. Gynecol. 211, 41.e41–e48 (2014).
Mercuri, E. et al. Neonatal Neurological Examination in Infants with Hypoxic Ischaemic Encephalopathy: Correlation with MRI Findings. Neuropediatrics 30, 83–89 (2007).
Laptook, A. R. et al. Outcome of Term Infants Using Apgar Scores at 10 min Following Hypoxic-Ischemic Encephalopathy. Pediatrics 124, 1619–1626 (2009).
Sarkar, S., Bhagat, I., Dechert, R. E. & Barks, J. D. Predicting death despite therapeutic hypothermia in infants with hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 95, F423–F428 (2010).
Author information
Authors and Affiliations
Contributions
DR, HC, ME: Substantial contributions to conception and design; HE, AG: Acquisition of data; DR, Analysis and interpretation of data and drafting the article or revising it critically for important intellectual content; DR, HE, ES, AG, HC, ME: Final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rallis, D., El-Shibiny, H., Szakmar, E. et al. Impact of persistent pulmonary hypertension on cerebral oxygenation in infants with neonatal encephalopathy. Pediatr Res 98, 203–209 (2025). https://doi.org/10.1038/s41390-024-03718-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03718-y