Abstract
Background
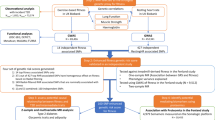
In type 1 diabetes, telomere length (TL) may predict complications and could be influenced by glycaemic control and physical activity, but its relationship with physical fitness in youths remains unexplored. The aim of the study was to assess the association between physical fitness and TL in youth with type 1 diabetes, both at baseline and one year later.
Methods
Eighty-three children and adolescents (aged 6–18 years; 44.6% girls) with type 1 diabetes from the Diactive-1 Cohort Study were involved in this study. Physical fitness was assessed using spirometry on a cycloergometer (i.e., peak oxygen consumption), dynamometry, and maximal isometric strength (one-repetition maximum [1RM]), and muscle power. Leucocyte TL was assessed using multiplex monochrome real-time quantitative polymerase chain reaction.
Results
Positive cross-sectional associations were identified between 1RM (unstandardized beta coefficient [B] = 0.042, 95% bias corrected and accelerated [BCa] confidence interval [CI] 0.012–0.069), muscle power (B = 0.056, 95% BCa CI 0.02–0.250), and overall physical fitness (B = 0.043, 95% BCa CI 0.015–0.071) with TL independent of maturation, glycated haemoglobin, and diabetes duration. However, no associations were observed one year later.
Conclusion
Higher levels of fitness, particularly muscle strength, may play a role in telomere dynamics in youth with type 1 diabetes, suggesting that strength training exercise could be beneficial.
Impact
-
This is the first study to examine cross-sectional and longitudinal perspectives on the correlation among muscle strength, peak oxygen consumption [VO2peak] and telomere length in youths with type 1 diabetes.
-
Higher physical fitness levels, as assessed by measures such as one-repetition maximum, muscle power, and overall physical fitness, are positively associated with telomere length in youths with type 1 diabetes.
-
Understanding this link could improve management strategies, prioritizing muscle strength training for better long-term health in type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
11 February 2025
The XML of this article was revised to reflect the author first and surname correctly.
References
Turner, K., Vasu, V. & Griffin, D. Telomere biology and human phenotype. Cells 8, 73 (2019).
Greider, C. W. & Blackburn, E. H. The telomere terminal transferase of tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 (1987).
Demanelis, K. et al. Determinants of telomere length across human tissues. Science 369, eaaz6876 (2020).
Sfeir, A. & De Lange, T. Removal of shelterin reveals the telomere end-protection problem. Science 336, 593–597 (2012).
Chakravarti, D., LaBella, K. A. & DePinho, R. A. Telomeres: history, health, and hallmarks of aging. Cell 184, 306–322 (2021).
Cheng, F. et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 9, 117–126 (2021).
Wang, J. et al. Association between telomere length and diabetes mellitus: a meta-analysis. J. Int. Med. Res. 44, 1156–1173 (2016).
Fyhrquist, F., Tiitu, A., Saijonmaa, O., Forsblom, C. & Groop, P-H. FinnDiane Study Group Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J. Intern. Med. 267, 278–286 (2010).
Januszewski, A. S. et al. Shorter telomeres in adults with type 1 diabetes correlate with diabetes duration, but only weakly with vascular function and risk factors. Diabetes Res. Clin. Pract. 117, 4–11 (2016).
Uziel, O. et al. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp. Gerontol. 42, 971–978 (2007).
Valente, C. et al. Effect of physical activity and exercise on telomere length: systematic review with meta‐analysis. J. Am. Geriatr. Soc. 69, 3285–3300 (2021).
Latifovic, L., Peacock, S. D., Massey, T. E. & King, W. D. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. Cancer Epidemiol. Biomark. Prev. 25, 374–380 (2016).
Sillanpää, E., Törmäkangas, T., Rantanen, T., Kaprio, J. & Sipilä, S. Does telomere length predict decline in physical functioning in older twin sisters during an 11-year follow-up? AGE 38, 34 (2016).
Song, Z. et al. Lifestyle impacts on the aging‐associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell 9, 607–615 (2010).
Von Känel R., Bruwer E. J., Hamer M., De Ridder J. H., Malan L. Association between objectively measured physical activity, chronic stress and leukocyte telomere length. J. Sports Med. Phys. Fitness. 57, (2017). https://doi.org/10.23736/S0022-4707.16.06426-4.
Schellnegger, M., Lin, A. C., Hammer, N. & Kamolz, L. P. Physical activity on telomere length as a biomarker for aging: a systematic review. Sports Med. Open 8, 111 (2022).
García-Hermoso, A., Ramírez-Campillo, R. & Izquierdo, M. Is muscular fitness associated with future health benefits in children and adolescents? A systematic review and meta-analysis of longitudinal studies. Sports Med. 49, 1079–1094 (2019).
García-Hermoso, A., Ramírez-Vélez, R., García-Alonso, Y., Alonso-Martínez, A. M. & Izquierdo, M. Association of cardiorespiratory fitness levels during youth with health risk later in life: a systematic review and meta-analysis. JAMA Pediatr. 174, 952 (2020).
Huerta-Uribe, N., Ramírez-Vélez, R., Izquierdo, M. & García-Hermoso, A. Association between physical activity, sedentary behavior and physical fitness and glycated hemoglobin in youth with type 1 diabetes: a systematic review and meta-analysis. Sports Med. 53, 111–123 (2023).
Denham, J. & Sellami, M. Exercise training increases telomerase reverse transcriptase gene expression and telomerase activity: a systematic review and meta-analysis. Ageing Res. Rev. 70, 101411 (2021).
Lin, X., Zhou, J. & Dong, B. Effect of different levels of exercise on telomere length: a systematic review and meta-analysis. J. Rehabil. Med. 51, 473–478 (2019).
Paltoglou, G. et al. A comprehensive, multidisciplinary, personalized, lifestyle intervention program is associated with increased leukocyte telomere length in children and adolescents with overweight and obesity. Nutrients 13, 2682 (2021).
Sánchez-González, J. L. et al. Effects of physical exercise on telomere length in healthy adults: systematic review, meta-analysis, and meta-regression. JMIR Public Health Surveill. 10, e46019 (2024).
Song, S., Lee, E. & Kim, H. Does exercise affect telomere length? a systematic review and meta-analysis of randomized controlled trials. Medicina 58, 242 (2022).
Kozieł, S. M. & Malina, R. M. Modified maturity offset prediction equations: validation in independent longitudinal samples of boys and girls. Sports Med. 48, 221–236 (2018).
ElSayed, N. A. et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes—2024. Diabetes Care 47, S52–S76 (2024).
Ojeda-Rodríguez, A. et al. Association between favourable changes in objectively measured physical activity and telomere length after a lifestyle intervention in pediatric patients with abdominal obesity. Appl. Physiol. Nutr. Metab. 46, 205–212 (2021).
Blair, R. C. & Higgins, J. J. Comparison of the power of the paired samples t test to that of Wilcoxon’s signed-ranks test under various population shapes. Psychol. Bull. 97, 119–128 (1985).
Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30, 377–399 (2011).
Sun, Y., Fang, J., Wan, Y., Su, P. & Tao, F. Association of early-life adversity with measures of accelerated biological aging among children in China. JAMA Netw. Open 3, e2013588 (2020).
Hiam, D. et al. Aerobic capacity and telomere length in human skeletal muscle and leukocytes across the lifespan. Aging 12, 359–369 (2020).
Åström, M. J. et al. Telomere length and physical performance among older people—the Helsinki Birth Cohort Study. Mech. Ageing Dev. 183, 111145 (2019).
Buttet, M. et al. Effect of a lifestyle intervention on telomere length: a systematic review and meta-analysis. Mech. Ageing Dev. 206, 111694 (2022).
Almuraikhy, S. et al. Impact of moderate physical activity on inflammatory markers and telomere length in sedentary and moderately active individuals with varied insulin sensitivity. J. Inflamm. Res. 16, 5427–5438 (2023).
Powers, S. K., Goldstein, E., Schrager, M. & Ji, L. L. Exercise training and skeletal muscle antioxidant enzymes: an update. Antioxidants 12, 39 (2022).
Ma, J. et al. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care a randomized trial. JAMA Intern. Med. 173, 113–121 (2013).
Paltoglou, G. et al. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre- and early-pubertal normal-weight and obese boys. Endocrine 55, 925–933 (2017).
Arsenis, N. C., You, T., Ogawa, E. F., Tinsley, G. M. & Zuo, L. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget 8, 45008–45019 (2017).
López-Gil, J. F., Ramírez-Vélez, R., Izquierdo, M. & García-Hermoso, A. Handgrip strength and its relationship with white blood cell count in U.S. adolescents. Biology 10, 884 (2021).
Tuttle, C. S. L., Thang, L. A. N. & Maier, A. B. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res. Rev. 64, 101185 (2020).
Kadi, F. & Ponsot, E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand. J. Med Sci. Sports 20, 39–48 (2010).
Masschelein, E. et al. Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet. Muscle 10, 21 (2020).
Daniali, L. et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 (2013).
Acknowledgements
The technical assistance of Veronica Ciaurriz from the Department of Nutrition, Food Sciences and Physiology and Center for Nutrition and Research at the University of Navarra is fully acknowledged.
Funding
This study was funded by grant PI21/01238 from the Instituto de Salud Carlos III (Spain) and by CIBER of Obesity Physiology and Nutrition (CB12/03/30002). The project that gave rise to these results received the support of a fellowship form “Instituto de Salud Carlos III” granted to Jacinto Muñoz-Pardeza (FI22/00329). Dr. Ana Ojeda-Rodriguez is the recipient of a Sara Borrell grant (CD21/00099) from the Instituto de Salud Carlos III. The origin of obtaining results was supported by a scholarship from the “la Caixa” Foundation awarded to Nidia Huerta Uribe (DNI 11780038).
Author information
Authors and Affiliations
Contributions
M.J.C.G., SBZ, E.B.S., and M.I. They were involved in the conception, design and conduct of the study. A.G.H, J.L.G, J.M.P. were involved in the statistical analyses and interpretation of the results obtained. A.M.D. and A.O.R. oversaw executing the necessary protocols to obtain the telomere length and reviewed and approved the final version of the paper. I.H.A and N.H.U managed the physical evaluations and interpretation of the metabolic variables. J.M.P. wrote the first draft of the manuscript, and A.G.H., J.L.G., and M.I. reviewed, edited, and approved the final version of the paper. A.G.H is the guarantee of the work and, as such, had full access to all the data of the study, assuming full responsibility for it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muñoz-Pardeza, J., López-Gil, J.F., Huerta-Uribe, N. et al. Is physical fitness associated with leucocyte telomere length in youth with type 1 diabetes?. Pediatr Res 97, 2354–2359 (2025). https://doi.org/10.1038/s41390-024-03732-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03732-0
This article is cited by
-
Invited Commentary: “Is physical fitness associated with leukocyte telomere length in youth with type 1 diabetes?”
Pediatric Research (2025)
-
Telomere length in youth with type 1 diabetes and the role of physical fitness
Pediatric Research (2025)