Abstract
Background
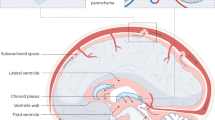
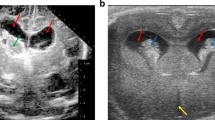
Post-hemorrhagic hydrocephalus (PHH) is a severe complication in premature infants following intraventricular hemorrhage (IVH). It is characterized by abnormal cerebrospinal fluid (CSF) accumulation, disrupted CSF dynamics, and elevated intracranial pressure (ICP), leading to significant neurological impairments.
Objective
This review provides an overview of recent molecular insights into the pathophysiology of PHH and evaluates emerging therapeutic approaches aimed at addressing its underlying mechanisms.
Methods
Recent studies were reviewed, focusing on molecular and cellular mechanisms implicated in PHH, including neuroinflammatory pathways, immune mediators, and regulatory genes. The potential of advanced technologies such as whole genome/exome sequencing, proteomics, epigenetics, and single-cell transcriptomics to identify key molecular targets was also analyzed.
Results
PHH has been strongly linked to neuroinflammatory processes triggered by the degradation of blood byproducts. These processes involve cytokines, chemokines, the complement system, and other immune mediators, as well as regulatory genes and epigenetic mechanisms. Current treatments, primarily surgical CSF diversion, do not address the underlying molecular pathology. Emerging therapies, such as mesenchymal stem cell-based interventions, show promise in modulating immune responses and mitigating neurological damage. However, concerns about the safety of these novel approaches in neonatal populations and their potential effects on brain development remain unresolved.
Conclusions
Advanced molecular tools and emerging therapies have the potential to transform the treatment of PHH by targeting its underlying pathophysiology. Further research is needed to validate these approaches, enhance their safety profiles, and improve outcomes for infants with PHH.
Impact statement
-
1.
This review elucidates the molecular complexities of post-hemorrhagic hydrocephalus (PHH) by examining specific immune pathways and their impact on disease pathogenesis and progression.
-
2.
It outlines the application of genomic, epigenomic, and proteomic technologies to identify critical molecular targets in PHH, setting the stage for innovative, targeted therapeutic approaches that could improve the outcomes of neonates affected by PHH.
-
3.
It discusses the potential of gene and stem cell therapies in treating PHH, offering non-surgical alternatives and focusing on the underlying neuroinflammatory mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Rekate, H. L. A contemporary definition and classification of hydrocephalus. Semin Pediatr. Neurol. 16, 9–15 (2009).
Kahle, K. T., Kulkarni, A. V., Limbrick, D. D. Jr. & Warf, B. C. Hydrocephalus in children. Lancet 387, 788–799 (2016).
Isaacs, A. M. et al. Age-specific global epidemiology of hydrocephalus: systematic review, metanalysis and global birth surveillance. PLoS One 13, e0204926 (2018).
Dewan, M. C. et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J. Neurosurg. 1–15 https://doi.org/10.3171/2017.10.JNS17439 (2018).
O’Hayon, B. B., Drake, J. M., Ossip, M. G., Tuli, S. & Clarke, M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr. Neurosurg. 29, 245–249 (1998).
Dandy, W. E. & Blackfan, K. D. An experimental and clinical study of internal hydrocephalus. J. Am. Med. Assoc. 61, 2216–2217 (1913).
Aronyk, K. E. The history and classification of hydrocephalus. Neurosurg. Clin. N. Am. 4, 599–609 (1993).
Mori, K. in Hydrocephalus: pathogenesis and treatment (eds S. Matsumoto & N. Tamaki) 362–368 (Springer Verlag, 1991).
Raimondi, A. J. A unifying theory for the definition and classification of hydrocephalus. Childs Nerv. Syst. 10, 2–12 (1994).
Rekate, H. L. A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv. Syst. 27, 1535–1541 (2011).
Tully, H. M. & Dobyns, W. B. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur. J. Med. Genet. 57, 359–368 (2014).
Chu, S. M. et al. Neurological complications after neonatal bacteremia: the clinical characteristics, risk factors, and outcomes. PLoS One 9, e105294 (2014).
Li, L. et al. Association of bacteria with hydrocephalus in Ugandan infants. J. Neurosurg. Pediatr. 7, 73–87 (2011).
Volpe, J. J. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clin. Perinatol. 16, 387–411 (1989).
Lai, G. Y. et al. Global incidence proportion of intraventricular haemorrhage of prematurity: a meta-analysis of studies published 2010-2020. Arch. Dis. Child Fetal Neonatal Ed. 107, 513–519 (2022).
Ballabh, P. & de Vries, L. S. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat. Rev. Neurol. 17, 199–214 (2021).
Adams-Chapman, I., Hansen, N. I., Stoll, B. J., Higgins, R. & Network, N. R. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121, e1167–1177 (2008).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Limbrick, D. D. Jr. et al. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J. Neurosurg. Pediatr. 6, 224–230 (2010).
Murphy, B. P. et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch. Dis. Child Fetal Neonatal Ed. 87, F37–F41 (2002).
Vassilyadi, M., Tataryn, Z., Shamji, M. F. & Ventureyra, E. C. Functional outcomes among premature infants with intraventricular hemorrhage. Pediatr. Neurosurg. 45, 247–255 (2009).
Radic, J. A., Vincer, M. & McNeely, P. D. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J. Neurosurg. Pediatr. 15, 580–588 (2015).
Hollebrandse, N. L. et al. School-age outcomes following intraventricular haemorrhage in infants born extremely preterm. Arch. Dis. Child Fetal Neonatal Ed. 106, 4–8 (2021).
Riva-Cambrin, J. et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J. Neurosurg. Pediatr. 9, 473–481 (2012).
Wellons, J. C. et al. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J. Neurosurg. Pediatr. 4, 50–55 (2009).
Wellons, J. C. et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J. Neurosurg. Pediatr. 20, 19–29 (2017).
Drake, J. M., Kulkarni, A. V. & Kestle, J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv. Syst. 25, 467–472 (2009).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67, 588–593 (2010).
Kulkarni, A. V. et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J. Neurosurg. Pediatr. 18, 423–429 (2016).
Kulkarni, A. V. et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls. Clin. Artic. J. Neurosurg. Pediatr. 12, 334–338 (2013).
Wellons, J. C. et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J. Neurosurg. Pediatr. 20, 19 (2017).
Cizmeci, M. N. et al. Randomized controlled early versus late ventricular intervention study in posthemorrhagic ventricular dilatation: outcome at 2 years. J. Pediatr. 226, 28–35 e23 (2020).
Luyt, K. et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 105, 466–473 (2020).
Paturu, M. et al. Does ventricle size contribute to cognitive outcomes in posthemorrhagic hydrocephalus? Role of early definitive intervention. J. Neurosurg. Pediatr. 29, 10–20 (2022).
Strahle, J. M. et al. Longitudinal CSF iron pathway proteins in posthemorrhagic hydrocephalus: associations with ventricle size and neurodevelopmental outcomes. Ann. Neurol. 90, 217–226 (2021).
Whitelaw, A. et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics 125, e852–858 (2010).
Garton, T., Hua, Y., Xiang, J., Xi, G. & Keep, R. F. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin. Ther. Targets 21, 1111–1122 (2017).
Kandula, V. et al. The role of blood product removal in intraventricular hemorrhage of prematurity: a meta-analysis of the clinical evidence. Childs Nerv. Syst. 38, 239–252 (2022).
Garton, T. et al. Intraventricular hemorrhage: the role of blood components in secondary injury and hydrocephalus. Transl. Stroke Res. 7, 447–451 (2016).
Strahle, J. et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 3, 25–38 (2012).
Koschnitzky, J. E. et al. Opportunities in posthemorrhagic hydrocephalus research: outcomes of the Hydrocephalus Association Posthemorrhagic Hydrocephalus Workshop. Fluids Barriers CNS 15, 11 (2018).
McAllister, J. P. et al. An update on research priorities in hydrocephalus: overview of the third National Institutes of Health-sponsored symposium “Opportunities for Hydrocephalus Research: Pathways to Better Outcomes. J. Neurosurg. 123, 1427–1438 (2015).
Del Bigio, M. R. & Di Curzio, D. L. Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS 13, 3 (2016).
Lolansen, S. D. et al. Posthemorrhagic hydrocephalus associates with elevated inflammation and CSF hypersecretion via activation of choroidal transporters. Fluids Barriers CNS 19, 62 (2022).
Otun, A. et al. Biochemical profile of human infant cerebrospinal fluid in intraventricular hemorrhage and post-hemorrhagic hydrocephalus of prematurity. Fluids Barriers CNS 18, 62 (2021).
Garcia-Bonilla, M. et al. Pro-inflammatory cerebrospinal fluid profile of neonates with intraventricular hemorrhage: clinical relevance and contrast with CNS infection. Fluids Barriers CNS 21, 17 (2024).
Lolansen, S. D. et al. Inflammatory markers in cerebrospinal fluid from patients with hydrocephalus: a systematic literature review. Dis. Markers 2021, 8834822 (2021).
Isaacs, A. M. & Limbrick, D. D. in Cerebrospinal Fluid Disorders 47–70 (Springer, Cham, 2019).
Habiyaremye, G. et al. Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus. Fluids Barriers CNS 14, 35 (2017).
Harris, C. A., Morales, D. M., Arshad, R., McAllister, J. P. 2nd & Limbrick, D. D. Jr. Cerebrospinal fluid biomarkers of neuroinflammation in children with hydrocephalus and shunt malfunction. Fluids Barriers CNS 18, 4 (2021).
Sival, D. A. et al. Neonatal high pressure hydrocephalus is associated with elevation of pro-inflammatory cytokines IL-18 and IFNgamma in cerebrospinal fluid. Cerebrospinal Fluid Res. 5, 21 (2008).
Schmitz, T. et al. Interleukin-1beta, interleukin-18, and interferon-gamma expression in the cerebrospinal fluid of premature infants with posthemorrhagic hydrocephalus-markers of white matter damage? Pediatr. Res. 61, 722–726 (2007).
Isaacs, A. M. et al. Microstructural periventricular white matter injury in post-hemorrhagic ventricular dilatation. Neurology 98, e364–e375 (2021).
Isaacs, A. M. et al. MR diffusion changes in the perimeter of the lateral ventricles demonstrate periventricular injury in post-hemorrhagic hydrocephalus of prematurity. Neuroimage Clin. 24, 102031 (2019).
Mayer, M. G. & Fischer, T. Microglia at the blood brain barrier in health and disease. Front. Cell Neurosci. 18, 1360195 (2024).
Yao, L. et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J. Neuroinflammation 10, 23 (2013).
Karimy, J. K., Reeves, B. C. & Kahle, K. T. Targeting TLR4-dependent inflammation in post-hemorrhagic brain injury. Expert Opin. Ther. Targets 24, 525–533 (2020).
Shao, F., Wang, X., Wu, H., Wu, Q. & Zhang, J. Microglia and neuroinflammation: crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front. Aging Neurosci. 14, 825086 (2022).
Zhang, Y. et al. Bliverdin reductase-A improves neurological function in a germinal matrix hemorrhage rat model. Neurobiol. Dis. 110, 122–132 (2018).
Cao, D. et al. CD36 regulates lipopolysaccharide-induced signaling pathways and mediates the internalization of Escherichia coli in cooperation with TLR4 in goat mammary gland epithelial cells. Sci. Rep. 6, 23132 (2016).
Zhao, X., Grotta, J., Gonzales, N. & Aronowski, J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke 40, S92–S94 (2009).
Chen, Q. et al. Simvastatin promotes hematoma absorption and reduces hydrocephalus following intraventricular hemorrhage in part by upregulating CD36. Transl. Stroke Res. 8, 362–373 (2017).
Yano, M. et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ. Res. 100, 1442–1451 (2007).
Ibrahim Fouad, G. Sulforaphane, an Nrf-2 agonist, modulates oxidative stress and inflammation in a rat model of cuprizone-induced cardiotoxicity and hepatotoxicity. Cardiovasc. Toxicol. 23, 46–60 (2023).
Zhao, X. et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem 133, 144–152 (2015).
Olsson, M., Nilsson, A. & Oldenborg, P. A. Dose-dependent inhibitory effect of CD47 in macrophage uptake of IgG-opsonized murine erythrocytes. Biochem. Biophys. Res. Commun. 352, 193–197 (2007).
Ye, F., Hua, Y., Keep, R. F., Xi, G. & Garton, H. J. L. CD47 blocking antibody accelerates hematoma clearance and alleviates hydrocephalus after experimental intraventricular hemorrhage. Neurobiol. Dis. 155, 105384 (2021).
Tao, C., Keep, R. F., Xi, G. & Hua, Y. CD47 blocking antibody accelerates hematoma clearance after intracerebral hemorrhage in aged rats. Transl. Stroke Res. 11, 541–551 (2020).
Robert, S. M. et al. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell 186, 764–785.e721 (2023).
Peng, J. et al. Toll-like receptor 4-mediated microglial inflammation exacerbates early white matter injury following experimental subarachnoid hemorrhage. J. Neurochem. 166, 280–293 (2023).
Karimy, J. K. et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 23, 997–1003 (2017).
Lin, T. et al. Pharmacological inhibition of TLR4-NF-kappaB signaling by TAK-242 attenuates hydrocephalus after intraventricular hemorrhage. Int. Immunopharmacol. 103, 108486 (2022).
Gurung, P. et al. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci. Rep. 5, 14488 (2015).
Qi, H. M., Cao, Q. & Liu, Q. TLR4 regulates vascular smooth muscle cell proliferation in hypertension via modulation of the NLRP3 inflammasome. Am. J. Transl. Res. 13, 314–325 (2021).
Janeway C. A., et al. in Immunobiology: The Immune System in Health and Disease (New York: Garland Science, 2021).
Alawieh, A., Langley, E. F., Weber, S., Adkins, D. & Tomlinson, S. Identifying the role of complement in triggering neuroinflammation after traumatic brain injury. J. Neurosci. 38, 2519–2532 (2018).
Alshareef, M. et al. A role of complement in the pathogenic sequelae of mouse neonatal germinal matrix hemorrhage. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23062943 (2022).
Foreman, K. E. et al. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 94, 1147–1155 (1994).
Hatchell, D. et al. A role for P-selectin and complement in the pathological sequelae of germinal matrix hemorrhage. J. Neuroinflammation 20, 143 (2023).
Kuo, L. T. & Huang, A. P. The pathogenesis of hydrocephalus following aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22095050 (2021).
Luo, J. TGF-beta as a key modulator of astrocyte reactivity: disease relevance and therapeutic implications. Biomedicines 10 https://doi.org/10.3390/biomedicines10051206 (2022).
Cherian, S., Whitelaw, A., Thoresen, M. & Love, S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 14, 305–311 (2004).
Cao, Y. et al. Metformin alleviates delayed hydrocephalus after intraventricular hemorrhage by inhibiting inflammation and fibrosis. Transl. Stroke Res. 14, 364–382 (2023).
Douglas-Escobar, M. & Weiss, M. D. Biomarkers of brain injury in the premature infant. Front. Neurol. 3, 185 (2012).
Douglas, M. R. et al. High CSF transforming growth factor beta levels after subarachnoid haemorrhage: association with chronic communicating hydrocephalus. J. Neurol. Neurosurg. Psychiatry 80, 545–550 (2009).
Botfield, H. et al. Decorin prevents the development of juvenile communicating hydrocephalus. Brain 136, 2842–2858 (2013).
Zhan, C. et al. Decreased MiR-30a promotes TGF-beta1-mediated arachnoid fibrosis in post-hemorrhagic hydrocephalus. Transl. Neurosci. 11, 60–74 (2020).
Chen, H., Chen, L., Xie, D. & Niu, J. Protective effects of transforming growth factor-beta1 knockdown in human umbilical cord mesenchymal stem cells against subarachnoid hemorrhage in a rat model. Cerebrovasc. Dis. 49, 79–87 (2020).
Isaacs, A. M. et al. Immune activation during Paenibacillus brain infection in African infants with frequent cytomegalovirus co-infection. iScience 24, 102351 (2021).
Wang, L. et al. Progress in research on TLR4-mediated inflammatory response mechanisms in brain injury after subarachnoid hemorrhage. Cells 11 https://doi.org/10.3390/cells11233781 (2022).
Johnsen, L. O., Friis, K. A. & Damkier, H. H. In vitro investigation of the effect of proinflammatory cytokines on mouse choroid plexus membrane transporters Ncbe and NKCC1. Fluids Barriers CNS 20, 71 (2023).
Banerjee, S., Biehl, A., Gadina, M., Hasni, S. & Schwartz, D. M. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77, 521–546 (2017).
Solimani, F., Meier, K. & Ghoreschi, K. Emerging topical and systemic JAK inhibitors in dermatology. Front. Immunol. 10, 2847 (2019).
Tanaka, Y., Luo, Y., O’Shea, J. J. & Nakayamada, S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat. Rev. Rheumatol. 18, 133–145 (2022).
Wang, Y. et al. SOCS1/JAK2/STAT3 axis regulates early brain injury induced by subarachnoid hemorrhage via inflammatory responses. Neural Regen. Res. 16, 2453–2464 (2021).
Ben Haim, L. et al. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 35, 2817–2829 (2015).
Ceyzeriat, K., Abjean, L., Carrillo-de Sauvage, M. A., Ben Haim, L. & Escartin, C. The complex STATes of astrocyte reactivity: how are they controlled by the JAK-STAT3 pathway? Neuroscience 330, 205–218 (2016).
Gialeli, A. et al. The miRNA transcriptome of cerebrospinal fluid in preterm infants reveals the signaling pathways that promote reactive gliosis following cerebral hemorrhage. Front. Mol. Neurosci. 16, 1211373 (2023).
Nobuta, H. et al. STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann. Neurol. 72, 750–765 (2012).
Dunn, J. F. & Isaacs, A. M. The impact of hypoxia on blood-brain, blood-CSF and CSF-brain barriers. J. Appl. Physiol. https://doi.org/10.1152/japplphysiol.00108.2020 (2021).
Karimy, J. K. et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat. Rev. Neurol. 16, 285–296 (2020).
Robert, S. M. et al. Inflammatory hydrocephalus. Childs Nerv. Syst. 37, 3341–3353 (2021).
Crack, P. J. et al. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflammation 11, 37 (2014).
Lummis, N. C. et al. LPA(1/3) overactivation induces neonatal posthemorrhagic hydrocephalus through ependymal loss and ciliary dysfunction. Sci. Adv. 5, eaax2011 (2019).
Yung, Y. C. et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 3, 99ra87 (2011).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20133328 (2019).
Cheng, S. et al. Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res. Bull. 127, 226–233 (2016).
Groslambert, M. & Py, B. F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 11, 359–374 (2018).
Zhang, Z. et al. NLRP3 inflammasome-mediated choroid plexus hypersecretion contributes to hydrocephalus after intraventricular hemorrhage via phosphorylated NKCC1 channels. J. Neuroinflammation 19, 163 (2022).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Nagra, G. et al. Elevated CSF outflow resistance associated with impaired lymphatic CSF absorption in a rat model of kaolin-induced communicating hydrocephalus. Cerebrospinal Fluid Res. 7, 4 (2010).
Eide, P. K. et al. Intrathecal contrast-enhanced magnetic resonance imaging of cerebrospinal fluid dynamics and glymphatic enhancement in idiopathic normal pressure hydrocephalus. Front. Neurol. 13, 857328 (2022).
Ringstad, G. et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3 https://doi.org/10.1172/jci.insight.121537 (2018).
Jacobsen, H. H. et al. In vivo evidence for impaired glymphatic function in the visual pathway of patients with normal pressure hydrocephalus. Investig. Ophthalmol. Vis. Sci. 61, 24 (2020).
Sevensky, R., Newville, J. C., Tang, H. L., Robinson, S. & Jantzie, L. L. Cumulative damage: cell death in posthemorrhagic hydrocephalus of prematurity. Cells 10 https://doi.org/10.3390/cells10081911 (2021).
Ding, Y. et al. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp. Neurol. 320, 113003 (2019).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Hablitz, L. M. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 5, eaav5447 (2019).
Jiang-Xie, L. F. et al. Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance. Nature 627, 157–164 (2024).
Murdock, M. H. et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature 627, 149–156 (2024).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Gomolka, R. S. et al. Loss of aquaporin-4 results in glymphatic system dysfunction via brain-wide interstitial fluid stagnation. Elife 12 https://doi.org/10.7554/eLife.82232 (2023).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4, 147ra111 (2012).
Fang, Y. et al. Pituitary adenylate cyclase-activating polypeptide attenuates brain edema by protecting blood-brain barrier and glymphatic system after subarachnoid hemorrhage in rats. Neurotherapeutics 17, 1954–1972 (2020).
Del Puerto, A. et al. Kidins220 deficiency causes ventriculomegaly via SNX27-retromer-dependent AQP4 degradation. Mol. Psychiatry 26, 6411–6426 (2021).
Botello-Smith, W. M. et al. A mechanism for the activation of the mechanosensitive Piezo1 channel by the small molecule Yoda1. Nat. Commun. 10, 4503 (2019).
Choi, D. et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat. Neurosci. https://doi.org/10.1038/s41593-024-01604-8 (2024).
Choi, D. et al. ORAI1 activates proliferation of lymphatic endothelial cells in response to laminar flow through Kruppel-like factors 2 and 4. Circ. Res. 120, 1426–1439 (2017).
Choi, D. et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J. Clin. Investig. 127, 1225–1240 (2017).
Choi, D. et al. Piezo1-regulated mechanotransduction controls flow-activated lymphatic expansion. Circ. Res. 131, e2–e21 (2022).
Matrongolo, M. J. et al. Piezo1 agonist restores meningeal lymphatic vessels, drainage, and brain-CSF perfusion in craniosynostosis and aged mice. J. Clin. Investig. 134 https://doi.org/10.1172/JCI171468 (2023).
Ridone, P., Vassalli, M. & Martinac, B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys. Rev. 11, 795–805 (2019).
Doring, Y., Soehnlein, O. & Weber, C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 120, 736–743 (2017).
Fruh, A. et al. RNase A inhibits formation of neutrophil extracellular traps in subarachnoid hemorrhage. Front. Physiol. 12, 724611 (2021).
Liu, J., Zhang, S., Jing, Y. & Zou, W. Neutrophil extracellular traps in intracerebral hemorrhage: implications for pathogenesis and therapeutic targets. Metab. Brain Dis. 38, 2505–2520 (2023).
Zhang, Q. et al. Neutrophil extracellular trap-mediated impairment of meningeal lymphatic drainage exacerbates secondary hydrocephalus after intraventricular hemorrhage. Theranostics 14, 1909–1938 (2024).
Pavan, C. et al. DNase treatment prevents cerebrospinal fluid block in early experimental pneumococcal meningitis. Ann. Neurol. 90, 653–669 (2021).
Filipczak, N. et al. Antibody-modified DNase I micelles specifically recognize the neutrophil extracellular traps (NETs) and promote their degradation. J. Control Release 354, 109–119 (2023).
Gu, L. et al. Integrating network pharmacology and transcriptomic omics reveals that akebia saponin D attenuates neutrophil extracellular traps-induced neuroinflammation via NTSR1/PKAc/PAD4 pathway after intracerebral hemorrhage. FASEB J. 38, e23394 (2024).
Kang, L. et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 11, 2488 (2020).
Kim, S. W., Lee, H., Lee, H. K., Kim, I. D. & Lee, J. K. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol. Commun. 7, 94 (2019).
Sadegh, C. et al. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron 111, 1591–1608.e1594 (2023).
Purohit, D. et al. Human cord blood derived unrestricted somatic stem cells restore aquaporin channel expression, reduce inflammation and inhibit the development of hydrocephalus after experimentally induced perinatal intraventricular hemorrhage. Front. Cell Neurosci. 15, 633185 (2021).
Garcia-Bonilla, M. et al. Neocortical tissue recovery in severe congenital obstructive hydrocephalus after intraventricular administration of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 11, 121 (2020).
Ahn, S. Y., Chang, Y. S., Sung, S. I. & Park, W. S. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose-escalation clinical trial. Stem Cells Transl. Med. 7, 847–856 (2018).
Jinnai, M. et al. A model of germinal matrix hemorrhage in preterm rat pups. Front. Cell Neurosci. 14, 535320 (2020).
Author information
Authors and Affiliations
Contributions
L.N.S. conducted the primary literature review and drafted the initial manuscript. A.V. synthesized molecular insights and contributed to the discussion of neuroinflammatory pathways in post-hemorrhagic hydrocephalus. M.M. provided expertise on epigenetic mechanisms and potential therapeutic targets. M.W. assisted with the conceptual framework and contributed to revising and refining the manuscript. J.A.P. and J.R.L. reviewed the clinical implications of neuroinflammation and potential treatments, ensuring relevance to neonatal care. M.G.-B. contributed insights on immune-mediated neuroinflammation. J.P.M. provided guidance on cerebrospinal fluid dynamics in PHH. K.C. reviewed discussions on advanced molecular tools, including transcriptomics and proteomics. R.K.W. and E.R.M. contributed expertise on genomic technologies and their translational applications. D.D.L. provided critical feedback on therapeutic approaches and future directions. A.M.I. conceived the review, coordinated contributions from all authors, and finalized the manuscript for submission. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schulz, L.N., Varghese, A., Michenkova, M. et al. Neuroinflammatory pathways and potential therapeutic targets in neonatal post-hemorrhagic hydrocephalus. Pediatr Res 97, 1345–1357 (2025). https://doi.org/10.1038/s41390-024-03733-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03733-z