Abstract
Background
This non-inferiority, cross-over study aims to evaluate a novel proof-of-concept portable respiratory support device specifically designed for low-resource settings. The device integrates a ventilation module and an oxygen concentrator.
Methods
We studied twelve 4-week-old piglets with a mean weight of 8.4 kg before and after oleic acid-induced acute respiratory distress syndrome (ARDS). In each condition, animals received 1-h pressure control ventilation using a conventional ventilator (Servo-i, Getinge, SE) and the experimental ventilator in random sequence. Arterial blood gas analysis was performed every half-hour to adjust the ventilator settings. The primary outcome was partial pressure of oxygen to FiO2 ratio (P/F) with a non-inferiority margin of 50 mmHg.
Results
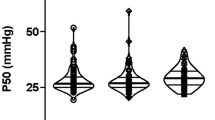
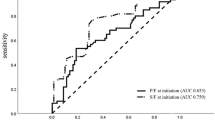
P/F did not differ significantly between the experimental and the control ventilation at baseline (459.6(30.9) vs 454.4(28.6) mmHg) and during ARDS condition (165.1(36.9) vs 182.5(48.4) mmHg). The upper 95% CI of the difference between P/F after ventilation using the control and the experimental ventilator was 37.3 and 44.1 mmHg during baseline and ARDS, respectively.
Conclusions
The experimental device was not inferior to a conventional ventilator during both baseline and ARDS conditions, suggesting that it can provide adequate treatment to infants with mild to moderate hypoxemic lung disease in resource-limited care settings.
Impact statement
-
This manuscript provides the results of a non-inferiority study that compared a novel proof-of-concept respiratory support device, integrating a ventilation module and an oxygen concentrator, specifically designed for respiratory support in low-resource settings, with a conventional pediatric intensive care ventilator in an oleic-acid model of acute lung injury. Our results showed that the experimental device was non-inferior to a conventional ventilator, suggesting that it can provide adequate treatment to infants with mild to moderate hypoxemic lung disease in resource-limited care settings. The developed solution can also be relevant for other applications, including home mechanical ventilation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Truog, R. D., Mitchell, C. & Daley G. Q. The toughest triage—Allocating ventilators in a pandemic. N. Engl. J. Med. 382, https://doi.org/10.1056/NEJMp2005689 (2020).
World Health Organization (WHO) and the International Committee of the Red Cross (ICRC). WHO-ICRC Basic Emergency Care: Approach to the Acutely Ill and Injured. (2018).
Madzimbamuto, F. D. Ventilators are not the answer in Africa. African Journal of Primary Health Care & Family Medicine. 12, https://doi.org/10.4102/phcfm (2020).
World Health Organization (WHO). Oxygen Therapy for Children. (2016).
World Health Organization (WHO) and United Nations Children’s Fund (UNICEF). WHO-UNICEF Technical Specifications and Guidance for Oxygen Therapy Devices. (2019).
Poletto, S. et al. Bubble CPAP respiratory support devices for infants in low-resource settings. Pediatr. Pulmonol. 58, 643–652 https://doi.org/10.1002/ppul.26258 (2023).
Park, S. & Suh, E. S. Home mechanical ventilation: Back to basics. Acute Crit. Care 35, 131–141 https://doi.org/10.4266/acc.2020.00514 (2020).
Sofia, P. et al. A novel CPAP device with an integrated oxygen concentrator for low resource countries: In vitro validation and usability test in field. IEEE Open J. Eng. Med Biol. https://doi.org/10.1109/OJEMB.2024.3413574 (2024).
Durbin, C. G. Jr How to come up with a good research question: Framing the hypothesis. Respir. Care 49, 1195–1198 (2004).
Bliss, P. L., McCoy, R. W. & Adams, A. B. A bench study comparison of demand oxygen delivery systems and continuous flow oxygen. Respir. Care 44, 925–931 (1999).
Putensen, C., Räsänen, J. & Downs, J. B. Effect of endogenous and inhaled nitric oxide on the ventilation-perfusion relationships in oleic-acid lung injury. Am. J. Respir. Crit. Care Med. 150, 330–336 https://doi.org/10.1164/ajrccm.150.2.8049811 (1994).
Schuster, D. P. ARDS: clinical lessons from the oleic acid model of acute lung injury. Am. J. Respir. Crit. Care Med. 149, 245–260 https://doi.org/10.1164/ajrccm.149.1.8111590 (1994).
Schuster, D. P. & Trulock, E. P. Correlation of changes in oxygenation, lung water and hemodynamics after oleic acid-induced acute lung injury in dogs. Crit. Care Med. 12, 1044–1048 https://doi.org/10.1097/00003246-198412000-00009 (1984).
Acknowledgements
We would like to thank Prof. Simone Cinquemani, M.Sc Andrea Caloni from the Mechanical Department, Prof. Paolo Rocco, Prof. Matteo Corno and Ing. Davide Bizzotto from the Electronic, Information and Bioengineering Department of Politecnico di Milano for their technical support; MD Daniele Trevisanuto from Padua University, Paolo Villani from the Istituto Ospedaliero Fondazione Poliambulanza of Brescia, MD Martin Langer from Emergency, Dott. Luciano Moccia from Day One Health, MD Barbara Tomasini and Stefano Zani from CUAMM-Medici con l’Africa for their fundamental contribution with their suggestions and feedback during the development of the project; Dott. Gregory Dajer and his team from MTTS-Asia for their support in the definition of the specifications of the prototype. Funding: Polisocial Award 2020, Project SAFER: “New technologies for safe and effective respiratorysupport during emergencies and in low-resource settings”.
Author information
Authors and Affiliations
Contributions
S.P. contributed to the study design, the development of the experimental device, the experimental activity and data analysis and drafted the first version of the manuscript; J.D. contributed to the study design, the experimental activity, data interpretation, critically revised the manuscript and approved the final manuscript as submitted; A.M. and C.M. contributed to the development of the experimental device and data collection and approved the final manuscript as submitted; F.F. and X.B. to the experimental activity and approved the final manuscript as submitted, E.Z. contributed to the study design and data analysis, critically revised the manuscript and approved the final manuscript as submitted; W.H. contributed to the study design, participated and supervised to the experimental activity and data interpretation, critically revised the manuscript and approved the final manuscript as submitted; R.L.D. contributed to the study design, supervised the development of the experimental device, participated to the experimental activity, critically revised the manuscript and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the University of Geneva Animal Ethics Committee of the Canton of Geneva (GE184). Animals were cared for in accordance with current Swiss animal protection laws (LPA, RS455).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poletto, S., Diaper, J., Montanarini, A. et al. Experimental validation of a novel portable device integrating an oxygen concentrator and a ventilation module for patients with ALI/ARDS in low resource countries: a cross-over non-inferiority trial. Pediatr Res 98, 100–106 (2025). https://doi.org/10.1038/s41390-024-03792-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-024-03792-2
This article is cited by
-
“An opportunity to help neonates and small infants to breath in low and middle-income countries”
Pediatric Research (2025)