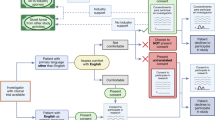
Abstract
Over one-fifth of US households speak a language other than English. While some studies have documented language-based disparities in pediatric clinical research, they are limited in scope and not representative of all US pediatric trials. Because language-based exclusion, if extensive, would limit the generalizability of the results of the research, we performed a systematic analysis of language-based eligibility criteria across all 4982 US pediatric interventional clinical trials registered on ClinicalTrials.gov from 2019 to 2022. We found that 70.0% [95% CI: 68.8–71.3%] of trials did not include any information about language while 23.4% [22.2–24.5%] had explicit English language requirements, of which only a minority (14.4%) included justifications for the limitation. Conversely, 6.6% [5.9–7.3%] of trials accommodated non-English languages. Trials with a posted protocol were more likely than all registered trials to include English language requirements (P < 0.00001). Trials with a federal sponsor, a behavioral intervention, or a focus on prevention or supportive care were more likely to report information about language when compared to all registered trials (P < 0.001), while trials with industry sponsors, drug or biological interventions, or a focus on treatment were less likely to do so (P < 0.001). Although modest, the percentage of trials requiring English decreased and the percentage offering language services increased from 2019 to 2022.
Impact Statement
-
Of 4982 US pediatric interventional studies registered on ClinicalTrials.gov from 2019 to 2022, 70.0% did not include any information about language while 23.4% explicitly included English language requirements. Of the trials requiring English, only 14.4% provided a justification for the requirement.
-
Trials with a posted protocol were more likely to require English than all registered trials.
-
Trials with industry sponsors, drug-based/biological interventions, or a treatment focus were less likely to mention language information than all registered trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The computer program and full data are available upon request.
References
U.S. Census Bureau. American Community Survey Reports: Language Use in the United States. https://www.census.gov/content/dam/Census/library/publications/2022/acs/acs-50.pdf (2019).
U.S. Census Bureau. American Community Survey Reports: Language Use in the United States. https://data.census.gov/table?q=DP02&y=2022 (2022).
Flores, G. & Tomany-Korman, S. C. The Language Spoken at Home and Disparities in Medical and Dental Health, Access to Care, and Use of Services in US Children. Pediatrics 121, e1703–e1714 (2008).
Do, V. et al. Exploring the lived experience of patients and families who speak language other than English (LOE) for healthcare: developing a qualitative study. Res. Involvement Engagement. 9, 49 (2023).
Muthukumar, A. V., Morrell, W. & Bierer, B. E. Evaluating the frequency of English language requirements in clinical trial eligibility criteria: A systematic analysis using clinicaltrials. Gov. PloS Med. 18, e1003758 (2021).
Frayne, S. M., Burns, R. B., Hardt, E. J., Rosen, A. K. & Moskowitz, M. A. The exclusion of non-English-speaking persons from research. J. Gen. Intern Med 11, 39–43 (1996).
Liss, J., Peloquin, D., Barnes, M. & Bierer, B. E. Applying civil rights law to clinical research: Title VI’s equal access mandate. J. Law Med. Ethics 50, 101–108 (2022).
Glickman, S. W. et al. Perspective: The case for research justice: inclusion of patients with limited English proficiency in clinical research. Acad. Med. 86, 389–393 (2011).
Chen, A. et al. Inclusion of Non–English-Speaking participants in Pediatric health research: A review. JAMA Pediatr. 177, 81–88 (2023).
Anwar, A., Dawson-Hahn, E., Lion, K. C., Jimenez, M. E. & Yun, K. Exclusion of families who speak languages other than English from Federally Funded Pediatric Trials. J. Pediatr. 262, 113597 (2023).
ClinicalTrials.Gov Protocol Registration Data Element Definitions for Interventional and Observational Studies. https://prsinfo.clinicaltrials.gov/definitions.html.
Capps, R., Fix, M. E., Ost, J. & Reardon-Anderson, J., Passel, J. S. The Health and Well-Being of Young Children of Immigrants. Urban Institute Nonprofit Social and Economic Policy Research; 2005. https://webarchive.urban.org/publications/311139.html. Published February 8, 2005.
Jaklevic, M. C. Researchers strive to recruit hard-hit minorities into COVID-19 vaccine trials. JAMA 324, 826–828 (2020).
Acknowledgements
We thank Ava Glazier (AG) and Molly Siegal (MS) for classifying language requirements and services as well as contributing to discussions about clarifying discrepancies.
Funding
Funding was provided by Harvard Catalyst (NCATS, NIH UL1TR002541) to support statistical consultation. The content is solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. A.V.M., B.E.B. conceived of the project and study design. A.V.M. performed the initial analysis and had unrestricted access to all data and prepared the first draft of the manuscript. R.J.G. performed the statistical analysis. K.M.S., and R.J.G., B.E.B. reviewed the data and analysis, and reviewed and edited the manuscript. All authors read and approved the final manuscript and take full responsibility for its content, including the accuracy of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent Statement
No patient consent was required for this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muthukumar, A.V., Shah, K.M., Glynn, R.J. et al. Persistent exclusion of non-English speakers in Pediatric research: a national analysis using ClinicalTrials.gov. Pediatr Res 98, 839–843 (2025). https://doi.org/10.1038/s41390-025-03845-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-03845-0