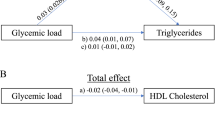
Abstract
Background
Central obesity is associated with an increased risk of hypertension and coronary heart disease. However, its molecular mechanisms have not yet been fully understood. This study aims to investigate lipids and proteins related to childhood central obesity, exploring the molecular mechanisms underlying this condition.
Methods
A case-control study was conducted, including a total of 169 children (aged 7–16 years, 53.25% male, and 74 children in the central obesity group). Plasma lipidomics were measured in all 169 children, and plasma proteomics was measured in 112 of these children. The transcriptomics and lipidomics of the mice’s liver were measured for normal feed and high-fat feed mice.
Results
Forty-six key lipids significantly associated with central obesity were identified, predominantly triglycerides (TAG), with a minority being diacylglycerols (DAG). Additionally, six key proteins, namely PLIN1, PLAT, ADH1A, ADH4, LEP, and INHB, were discovered, which may positively influence the central obesity phenotype by modulating levels of lipids such as TAG, DAG, LDL-C, and HDL-C. These proteins exhibited increased expression in the plasma of children with central obesity. Validation using mouse liver samples showed some overlapping differential lipids between mice and children, albeit minimal overlap in differential genes. This discrepancy may stem from inherent differences between transcriptomics and proteomics, species variations, and differing sampling sites.
Conclusions
PLIN1, PLAT, ADH1A, ADH4, LEP, and INHB are potential significant biomarkers for childhood central obesity and may influence the phenotype of childhood central obesity by modulating levels of lipids such as TAG, DAG, LDL-C, and HDL-C.
Impact
-
PLIN1, PLAT, ADH1A, ADH4, LEP, and INHB are potentially significant biomarkers for childhood central obesity and may influence the phenotype of childhood central obesity by modulating levels of lipids such as TAG, DAG, LDL-C, and HDL-C.
-
Research on integrating lipidomics and proteomics to elucidate the mechanisms of obesity, especially childhood central obesity, remains extremely limited. Our study filled this gap.
-
Our findings highlight potential biomarkers and therapeutic targets that could pave the way for new interventions and treatments to prevent central obesity in children and its harm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data is available from the corresponding author Xiaohua Liang (Email: xiaohualiang@hospital.cqmu.edu.cn, or liangxiaohua666@sina.com).
References
Han, J. C., Lawlor, D. A. & Kimm, S. Y. Childhood Obesity. Lancet 375, 1737–1748 (2010).
Lakshman, R., Elks, C. E. & Ong, K. K. Childhood Obesity. Circulation 126, 1770–1779 (2012).
World Health Organization. Taking Action on Childhood Obesity (World Health Organization, 2018).
Donahue, R., Bloom, E., Abbott, R., Reed, D. & Yano, K. Central Obesity and Coronary Heart Disease in Men. Lancet 329, 821–824 (1987).
Sahakyan, K. R. et al. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann. Intern. Med. 163, 827–835 (2015).
Bluher, M. et al. Dysregulation of the Peripheral and Adipose Tissue Endocannabinoid System in Human Abdominal Obesity. Diabetes 55, 3053–3060 (2006).
Elks, C. M. & Francis, J. Central Adiposity, Systemic Inflammation, and the Metabolic Syndrome. Curr. Hypertens. Rep. 12, 99–104 (2010).
Phillips, L. K. & Prins, J. B. The Link between Abdominal Obesity and the Metabolic Syndrome. Curr. Hypertens. Rep. 10, 156–164 (2008).
Abu-Farha, M. et al. Proteomics Analysis of Human Obesity Reveals the Epigenetic Factor Hdac4 as a Potential Target for Obesity. PloS One 8, e75342 (2013).
Choi, J. W. et al. Plasma Proteome Analysis in Diet‐Induced Obesity‐Prone and Obesity‐Resistant Rats. Proteomics 10, 4386–4400 (2010).
Hwang, H. et al. Proteomics Analysis of Human Skeletal Muscle Reveals Novel Abnormalities in Obesity and Type 2 Diabetes. Diabetes 59, 33–42 (2010).
Li, F. et al. Lipidomics Reveals a Link between Cyp1b1 and Scd1 in Promoting Obesity. J. proteome Res. 13, 2679–2687 (2014).
Rauschert, S. et al. Lipidomics Reveals Associations of Phospholipids with Obesity and Insulin Resistance in Young Adults. J. Clin. Endocrinol. Metab. 101, 871–879 (2016).
Tonks, K. T. et al. Skeletal Muscle and Plasma Lipidomic Signatures of Insulin Resistance and Overweight/Obesity in Humans. Obesity 24, 908–916 (2016).
Wakabayashi, I. Necessity of Both Waist Circumference and Waist-to-Height Ratio for Better Evaluation of Central Obesity. Metab. Syndr. Relat. Disord. 11, 189–194 (2013).
Yoo, E.-G. Waist-to-Height Ratio as a Screening Tool for Obesity and Cardiometabolic Risk. Korean J. Pediatrics 59, 425 (2016).
Dou, Y. et al. Waist-to-Height Ratio as a Screening Tool for Cardiometabolic Risk in Children and Adolescents: A Nationwide Cross-Sectional Study in China. BMJ Open 10, e037040 (2020).
Langfelder, P. & Horvath, S. Wgcna: An R Package for Weighted Correlation Network Analysis. BMC Bioinforma. 9, 1–13 (2008).
Ritchie, M. E. et al. Limma Powers Differential Expression Analyses for Rna-Sequencing and Microarray Studies. Nucleic Acids Res. 43, e47 (2015).
Wu, T. et al. Clusterprofiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2, 100141 (2021).
Szklarczyk, D. et al. The String Database in 2021: Customizable Protein–Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 49, D605–D612 (2021).
Consortium, U. Uniprot: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of Fold Change and Dispersion for Rna-Seq Data with Deseq2. Genome Biol. 15, 1–21 (2014).
Subramanian, A. et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl Acad. Sci. 102, 15545–15550 (2005).
Martínez, M. C. & Andriantsitohaina, R. Extracellular Vesicles in Metabolic Syndrome. Circ. Res. 120, 1674–1686 (2017).
Milbank, E., Martinez, M. C. & Andriantsitohaina, R. Extracellular Vesicles: Pharmacological Modulators of the Peripheral and Central Signals Governing Obesity. Pharmacol. Therapeutics 157, 65–83 (2016).
Carr, M. C. & Brunzell, J. D. Abdominal Obesity and Dyslipidemia in the Metabolic Syndrome: Importance of Type 2 Diabetes and Familial Combined Hyperlipidemia in Coronary Artery Disease Risk. J. Clin. Endocrinol. Metab. 89, 2601–2607 (2004).
Yu, L. et al. Association between Serum Magnesium and Blood Lipids: Influence of Type 2 Diabetes and Central Obesity. Br. J. Nutr. 120, 250–258 (2018).
Meng, X., Zou, D., Shi, Z., Duan, Z. & Mao, Z. Dietary Diacylglycerol Prevents High‐Fat Diet‐Induced Lipid Accumulation in Rat Liver and Abdominal Adipose Tissue. Lipids 39, 37–41 (2004).
Rudkowska, I. et al. Diacylglycerol: Efficacy and Mechanism of Action of an Anti‐Obesity Agent. Obes. Res. 13, 1864–1876 (2005).
Saito, S., Hernandez-Ono, A. & Ginsberg, H. N. Dietary 1, 3-Diacylglycerol Protects against Diet-Induced Obesity and Insulin Resistance. Metabolism 56, 1566–1575 (2007).
Teramoto, T. et al. Significant Effects of Diacylglycerol on Body Fat and Lipid Metabolism in Patients on Hemodialysis. Clin. Nutr. 23, 1122–1126 (2004).
Nicolaidou, K., Isoldi, K. K., Ramer, N. J. & Sarcona, A. The Role of Perilipins in the Development of Obesity and Obesity-Related Diseases. Top. Clin. Nutr. 31, 248–256 (2016).
Ramos-Lopez, O. et al. Prediction of Blood Lipid Phenotypes Using Obesity-Related Genetic Polymorphisms and Lifestyle Data in Subjects with Excessive Body Weight. Int. J. Genomics 2018, 1–10 (2018).
Song, W. et al. A Functional Variant in the Exon 5 of Plin1 Reduces Risk of Central Obesity by Possible Regulation of Lipid Storage. Biochem. Biophys. Res. Commun. 456, 896–900 (2015).
Keragala, C. B. & Medcalf, R. L. Plasminogen: An Enigmatic Zymogen. Blood, J. Am. Soc. Hematol. 137, 2881–2889 (2021).
Mutch, N. J., Wilson, H. M. & Booth, N. A. Plasminogen Activator Inhibitor-1 and Haemostasis in Obesity. Proc. Nutr. Soc. 60, 341–347 (2001).
Chen, R., Yan, J., Liu, P., Wang, Z. & Wang, C. Plasminogen Activator Inhibitor Links Obesity and Thrombotic Cerebrovascular Diseases: The Roles of Pai-1 and Obesity on Stroke. Metab. Brain Dis. 32, 667–673 (2017).
Duell, E. J. et al. Genetic Variation in Alcohol Dehydrogenase (Adh1a, Adh1b, Adh1c, Adh7) and Aldehyde Dehydrogenase (Aldh2), Alcohol Consumption and Gastric Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (Epic) Cohort. Carcinogenesis 33, 361–367 (2012).
Hurley, T. D. & Edenberg, H. J. Genes Encoding Enzymes Involved in Ethanol Metabolism. Alcohol Res. Curr. Rev. 34, 339 (2012).
Gautheron, J. et al. Adh1b, the Adipocyte-Enriched Alcohol Dehydrogenase, Plays an Essential, Cell-Autonomous Role in Human Adipogenesis. Proc. Natl Acad. Sci. 121, e2319301121 (2024).
Petrosino, J. M. et al. Paracardial Fat Remodeling Affects Systemic Metabolism through Alcohol Dehydrogenase 1. J. Clin. Invest. 131, e141799 (2021).
Havel, P. J. Role of Adipose Tissue in Body-Weight Regulation: Mechanisms Regulating Leptin Production and Energy Balance. Proc. Nutr. Soc. 59, 359–371 (2000).
Qadir, M. I. & Ahmed, Z. Lep Expression and Its Role in Obesity and Type-2 Diabetes. Crit. Rev. Eukaryotic Gene Expression 27, 47–51(2017).
Chabicovsky, M., Herkner, K. & Rossmanith, W. Overexpression of Activin Βc or Activin Βe in the Mouse Liver Inhibits Regenerative Deoxyribonucleic Acid Synthesis of Hepatic Cells. Endocrinology 144, 3497–3504 (2003).
Hashimoto, O. et al. Cdna Cloning and Expression of Human Activin Βe Subunit. Mol. Cell. Endocrinol. 194, 117–122 (2002).
Vejda, S. et al. Expression and Dimerization of the Rat Activin Subunits Beta~ C and Beta~ E: Evidence for the Formation of Novel Activin Dimers. J. Mol. Endocrinol. 28, 137–148 (2002).
Vejda, S. et al. Expression of Activins C and E Induces Apoptosis in Human and Rat Hepatoma Cells. Carcinogenesis 24, 1801–1809 (2003).
Deaton, A. M. et al. Rare Loss of Function Variants in the Hepatokine Gene Inhbe Protect from Abdominal Obesity. Nat. Commun. 13, 4319 (2022).
Sugiyama, M. et al. Inhibin Βe (Inhbe) Is a Possible Insulin Resistance-Associated Hepatokine Identified by Comprehensive Gene Expression Analysis in Human Liver Biopsy Samples. PloS One 13, e0194798 (2018).
Acknowledgements
The authors would like to acknowledge all the children and the staffs of the six elementary schools in Sichuan and Chongqing.
Funding
This work was supported by the Natural Science Foundation Project (No. 82373590), Joint Medical Research Project of Chongqing Municipal Health Commission and Science and Technology Bureau (2023MSXM036,2025ZDXM008), Program for Youth Innovation in Future Medicine from Chongqing Medical University (No.W0088), General Project of Clinical Medical Research from National Clinical Research Center for Child Health and Disorders (No. NCRCCHD-2022-GP-01), the Young and Middle-aged Medical Outstanding Expert Project of Chongqing Municipal Health Commission (No. 78), Major Health Project of Chongqing Science and Technology Bureau (No. CSTC2021jscx-gksb-N0001, CSTB2023NSCQ-MSX0181), Intelligent Medicine Project (No.ZHYX202109). The funders had no role in the whole study research process, including study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.Z.: Methodology, Writing - Review & Editing; F.C., F.C. and L.Y.: Investigation, Supervision; X.Z. and X.C.: Validation, Resources; Q.L. and Q.Z.: Writing – Original Draft, Writing - Review & Editing; X.H.: Conceptualization, Writing - Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Approval for this study was obtained from the Institutional Review Board of Chongqing Medical University. Children and their guardians were informed about the details of our research plan. Written informed consent was obtained from each child and their parent or guardian before their inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, C., An, X., Xiao, L. et al. Integrative multi-omics analysis reveals molecular signatures of central obesity in children. Pediatr Res 98, 1374–1385 (2025). https://doi.org/10.1038/s41390-025-03958-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-025-03958-6
This article is cited by
-
Integration of gut microbiome and lipid metabolism reveals the anti-cancer effects of pentadecanoic acid on bladder cancer
BMC Medicine (2025)
-
Multiomics strategy-based obesity biomarkers discovery for precision medicine
International Journal of Obesity (2025)