Abstract
Background
Genetic variants in T-box transcription factor 4 (TBX4) cause pulmonary hypertension (PH); however, there are diverse phenotypes with respect to the timing and severity of disease. Previous mouse studies demonstrated that germline TBX4 knockout is embryonic lethal, but knowledge gaps exist in how postnatal disruption of TBX4 signaling affects lung structure and PH.
Methods
A mouse model was used in which TBX4 was inactivated on day of life (DOL) 1. On DOL21, lung function was evaluated, and tissue was collected. Radial alveolar counts (RAC), vessel density, and right ventricular hypertrophy (RVH) were assessed. Downstream lung angiogenic (VEGF, KDR, and eNOS) and inflammatory mediators (TNF-a and IL-1) were measured.
Results
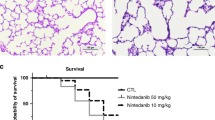
TBX4-deficient mice exhibited decreased RAC compared to controls (p < 0.05). Total lung resistance was increased, and total lung compliance was reduced in the TBX4-deficient group (p < 0.05, p < 0.01). Postnatal TBX4 deletion reduced lung vessel density (p < 0.001) and caused RVH (p < 0.01). Lung pro-angiogenic and inflammatory cytokine expression was reduced in TBX4-deficient mice.
Conclusion
Postnatal disruption of TBX4 signaling is sufficient to impair lung function, reduce alveolar and vascular growth, and cause RVH, which are associated with decreased lung expression of pro-angiogenic mediators but not enhanced inflammation.
Impact
-
TBX4 insufficiency is a rare genetic cause of pulmonary hypertension (PH) with poorly understood, variable phenotypes.
-
Postnatal disruption of TBX4 is sufficient to cause pulmonary vascular disease and impair lung development in infant mice. Although narrower in scope, we hypothesize that the late timing of TBX4 disruption plays a role in the severity of the lung phenotype.
-
Pro-angiogenic mediators (VEGF, KDR, and eNOS) and inflammatory cytokines (TNF-a and IL-1) are downregulated in the lungs of TBX4-deficient mice.
-
We speculate that greater insight into the mechanisms underlying TBX4-related PH may provide novel therapeutic targets for the management of TBX4 disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data supporting the findings of this study can be requested from the corresponding author by request.
References
Naeije, R., Richter, M. J. & Rubin, L. J. The physiological basis of pulmonary arterial hypertension. Eur. Respir. J. 59, 2102334 (2022).
Mocumbi, A. et al. Pulmonary hypertension. Nat. Rev. Dis. Prim. 10, 1 (2024).
Danhaive, O., Galambos, C., Lakshminrusimha, S. & Abman, S. H. Pulmonary hypertension in developmental lung diseases. Clin. Perinatol. 51, 217–235 (2024).
Welch, C. L. & Chung, W. K. Genetics and other omics in pediatric pulmonary arterial hypertension. Chest 157, 1287–1295 (2020).
Welch, C. L. et al. Defining the clinical validity of genes reported to cause pulmonary arterial hypertension. Genet. Med. 25, 100925 (2023).
Kerstjens-Frederikse, W. S. et al. TBX4 mutations (Small Patella Syndrome) are associated with childhood-onset pulmonary arterial hypertension. J. Med. Genet. 50, 500–506 (2013).
Haarman, M. G., Kerstjens-Frederikse, W. S. & Berger, R. M. F. TBX4 variants and pulmonary diseases: getting out of the ‘Box’. Curr. Opin. Pulm. Med. 26, 277–284 (2020).
Cebra-Thomas, J. A. et al. T-box gene products are required for mesenchymal induction of epithelial branching in the embryonic mouse lung. Dev. Dyn. 226, 82–90 (2003).
Papaioannou, V. E. & Silver, L. M. The T-box gene family. Bioessays 20, 9–19 (1998).
Chapman, D. L. et al. Expression of the t-box family genes, TBX1-TBX5, during early mouse development. Dev. Dyn. 206, 379–390 (1996).
Duboc, V. et al. TBX4 function during hindlimb development reveals a mechanism that explains the origins of proximal limb defects. Development 148, dev199580 (2021).
Bongers, E. M. et al. Mutations in the human TBX4 gene cause Small Patella Syndrome. Am. J. Hum. Genet. 74, 1239–1248 (2004).
Krause, A. et al. TBX5 AND TBX4 transcription factors interact with a new chicken PDZ-LIM protein in limb and heart development. Dev. Biol. 273, 106–120 (2004).
Thoré, P. et al. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur. Respir. J. 55, 1902340 (2020).
Galambos, C. et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur. Respir. J. 54, 1801965 (2019).
Zhu, N. et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ. Genom. Precis. Med. 11, e001887 (2018).
Mullen, M. P. Understanding genotype-phenotype correlations in patients with TBX4 mutations: new views inside and outside the box. Am. J. Respir. Crit. Care Med. 206, 1448–1449 (2022).
Prapa, M. et al. First genotype-phenotype study in TBX4 syndrome: gain-of-function mutations causative for lung disease. Am. J. Respir. Crit. Care Med. 206, 1522–1533 (2022).
Karolak, J. A. et al. Molecular function and contribution of TBX4 in development and disease. Am. J. Respir. Crit. Care Med. 207, 855–864 (2023).
Arora, R., Metzger, R. J. & Papaioannou, V. E. Multiple roles and interactions of TBX4 AND TBX5 in development of the respiratory system. PLoS Genet. 8, e1002866 (2012).
Naiche, L. A. & Papaioannou, V. E. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 130, 2681–2693 (2003).
Maddaloni, C. et al. Neonatal persistent pulmonary hypertension related to a novel TBX4 mutation: case report and review of the literature. Ital. J. Pediatr. 50, 41 (2024).
Flanagan, F. O. et al. An intronic variant in TBX4 in a single family with variable and severe pulmonary manifestations. NPJ Genom. Med. 8, 7 (2023).
Naiche, L. A. & Papaioannou, V. E. TBX4 is not required for hindlimb identity or post-bud hindlimb outgrowth. Development 134, 93–103 (2007).
Barst, R. J. et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 129, 1914–1923 (2014).
Emery, J. L. & Mithal, A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch. Dis. Child. 35, 544–547 (1960).
Bose, C. et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124, e450–e458 (2009).
Thurlbeck, W. M. Internal surface area and other measurements in emphysema. Thorax 22, 483–496 (1967).
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax 37, 572–579 (1982).
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 2-intrauterine and early postnatal lung growth. Thorax 37, 580–583 (1982).
de La Roque, E. D. et al. Effect of chronic hypoxia on pulmonary artery blood velocity in rats as assessed by electrocardiography-triggered three-dimensional time-resolved MR angiography. NMR Biomed. 24, 225–230 (2011).
Papaioannou, V. E. The T-box gene family: emerging roles in development, stem cells and cancer. Development 141, 3819–3833 (2014).
Karolak, J. A. et al. Complex compound inheritance of lethal lung developmental disorders due to disruption of the TBX-FGF pathway. Am. J. Hum. Genet. 104, 213–228 (2019).
Suhrie, K. et al. Neonatal lung disease associated with TBX4 mutations. J. Pediatr. 206, 286–292.e281 (2019).
German, K. et al. Identification of a deletion containing TBX4 in a neonate with acinar dysplasia by rapid exome sequencing. Am. J. Med. Genet. A 179, 842–845 (2019).
Szafranski, P. et al. Phenotypic expansion of TBX4 mutations to include acinar dysplasia of the lungs. Am. J. Med. Genet. A 170, 2440–2444 (2016).
Chelladurai, P. et al. Targeting histone acetylation in pulmonary hypertension and right ventricular hypertrophy. Br. J. Pharmacol. 178, 54–71 (2021).
Chelladurai, P. et al. Epigenetic reactivation of transcriptional programs orchestrating fetal lung development in human pulmonary hypertension. Sci. Transl. Med. 14, eabe5407 (2022).
Sakiyama, J., Yamagishi, A. & Kuroiwa, A. TBX4-FGF10 system controls lung bud formation during chicken embryonic development. Development 130, 1225–1234 (2003).
Karolak, J. A., Gambin, T., Szafranski, P. & Stankiewicz, P. Potential interactions between the TBX4-FGF10 and Shh-Foxf1 signaling during human lung development revealed using ChIP-seq. Respir. Res. 22, 26 (2021).
Yoshida, Y. et al. Genetic and functional analyses of TBX4 reveal novel mechanisms underlying pulmonary arterial hypertension. J. Mol. Cell. Cardiol. 171, 105–116 (2022).
Compernolle, V. et al. Loss of HIF-2alpha and Inhibition of VEGF Impair fetal lung maturation, whereas treatment with VEGF Prevents fatal respiratory distress in premature mice. Nat. Med. 8, 702–710 (2002).
Grover, T. R. et al. Intrauterine hypertension decreases lung VEGF expression and VEGF INhibition causes pulmonary hypertension in the ovine fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L508–L517 (2003).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L600–L607 (2000).
Le Cras, T. D., Markham, N. E., Tuder, R. M., Voelkel, N. F. & Abman, S. H. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L555–L562 (2002).
Domingo, L. T. et al. Novel use of riociguat in infants with severe pulmonary arterial hypertension unable to wean from inhaled nitric oxide. Front. Pediatr. 10, 1014922 (2022).
Giesinger, R. E., Stanford, A. H., Thomas, B., Abman, S. H. & McNamara, P. J. Safety and feasibility of riociguat therapy for the treatment of chronic pulmonary arterial hypertension in infancy. J. Pediatr. 255, 224–229.e221 (2023).
Katsura, H., Kobayashi, Y., Tata, P. R. & Hogan, B. L. M. IL-1 and TNFα contribute to the inflammatory niche to enhance alveolar regeneration. Stem Cell Rep. 12, 657–666 (2019).
Predescu, D. N., Mokhlesi, B. & Predescu, S. A. The impact of sex chromosomes in the sexual dimorphism of pulmonary arterial hypertension. Am. J. Pathol. 192, 582–594 (2022).
Funding
This work was supported by grant funding from the NIH (HL68702; SHA).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: Caroline F. Smith, Kathy L. Ding, Gregory J. Seedorf, Csaba Galambos, Steven H. Abman. Drafting the article or revising it critically for important intellectual content: Caroline F. Smith, Gregory J. Seedorf, Csaba Galambos, Steven H. Abman. Final approval of the version to be published: Caroline F. Smith, Kathy L. Ding, Gregory J. Seedorf, Csaba Galambos, Steven H. Abman.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith, C.F., Ding, K.L., Seedorf, G.J. et al. Postnatally induced TBX4 insufficiency confers pulmonary hypertension and impairs lung development in infant mice. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04127-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04127-5