Abstract
Background
This study investigates the gastrointestinal adverse events (AEs) associated with oral erythromycin, clarithromycin, and azithromycin in pediatric patients using the FAERS database.
Methods
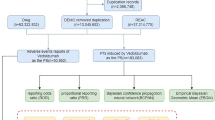
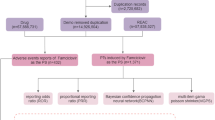
A retrospective pharmacovigilance analysis was conducted on FAERS reports from January 2004 to June 2024. Reports involving pediatric patients (<18 years) with gastrointestinal AEs linked to oral macrolides were analyzed. Disproportionality analysis using the reporting odds ratio (ROR) was performed to assess AE signals.
Results
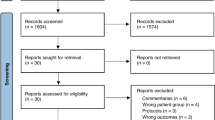
A total of 2179 AE reports were identified from the FAERS database in which oral erythromycin, clarithromycin, or azithromycin served as the primary suspect drugs, and among these, 705 (32.35%) reports involved gastrointestinal AEs. Azithromycin accounted for 57.45% of cases (ROR = 2.07), clarithromycin 36.17% (ROR = 2.10), and erythromycin 6.38% (ROR = 3.17). Vomiting, diarrhea, and nausea were the most frequently reported AEs. Several novel AE signals not listed in drug labels were detected, including lip swelling and gastric hemorrhage. The median onset time for AEs was 0 days for azithromycin and clarithromycin, and 3 days for erythromycin.
Conclusion
While most gastrointestinal AEs align with known drug profiles, newly identified signals warrant further investigation. Due to limitations of spontaneous reporting systems, future cohort studies are needed to validate these findings and enhance pediatric antibiotic safety.
Impact
-
This study examined gastrointestinal side effects in children taking three oral macrolide antibiotics (erythromycin, clarithromycin, and azithromycin) using data from the FAERS database.
-
Out of 2179 adverse event reports, nearly one-third involved gastrointestinal issues, with vomiting, diarrhea, and nausea being the most common.
-
Unexpected signals—such as lip swelling and gastric hemorrhage—were also identified, highlighting the need for further research to enhance the safety of these antibiotics in pediatric patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data supporting the findings of this study are not publicly available due to data privacy regulations but may be requested from the corresponding author upon reasonable request.
References
Antonakos, N., Giamarellos-Bourboulis, E. J. & Niederman, M. S. The role of macrolides in severe community-acquired pneumonia and the potential impact of macrolide-resistant Mycoplasma pneumoniae. Curr. Opin. Infect. Dis. 38, 190–197 (2025).
Gao, L. & Sun, Y. Laboratory diagnosis and treatment of Mycoplasma pneumoniae infection in children: a review. Ann. Med. 56, 2386636 (2024).
Nor Amdan, N. A., Shahrulzamri, N. A., Hashim, R. & Mohamad Jamil, N. Understanding the evolution of macrolides resistance: a mini review. J. Glob. Antimicrob. Resist. 38, 368–375 (2024).
Wiesner, A., Zagrodzki, P., Gawalska, A. & Pasko, P. Clinically important interactions of macrolides and tetracyclines with dietary interventions-a systematic review with meta-analyses. J. Antimicrob. Chemother. 79, 2762–2791 (2024).
Moja, L. et al. WHO’s essential medicines and AWaRe: recommendations on first- and second-choice antibiotics for empiric treatment of clinical infections. Clin. Microbiol. Infect. 30, S1–S51 (2024).
Li, D. & Wang, Y. Safety of azithromycin in pediatric infectious diseases: a clinical systematic review and meta-analysis. Transl. Pediatr. 10, 2594–2601 (2021).
Roberts, A. R. et al. Azithromycin for pediatric critical asthma: a multicenter retrospective cohort study. Hosp. Pediatr. 14, e254–e259 (2024).
Ukkonen, R. M., Renko, M. & Kuitunen, I. Azithromycin for acute bronchiolitis and wheezing episodes in children - a systematic review with meta-analysis. Pediatr. Res. 95, 1441–1447 (2024).
Ding, G. et al. Challenges in the treatment of pediatric Mycoplasma pneumoniae pneumonia. Eur. J. Pediatr. 183, 3001–3011 (2024).
Nakagawa, N. et al. Efficacy and safety of long-term macrolide therapy for non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis. Respir. Investig. 62, 1079–1087 (2024).
Ramos, S. F. et al. Moderate and serious adverse reactions to antimicrobials among hospitalized children: a systematic review. Br. J. Clin. Pharmacol. 90, 2092–2110 (2024).
Eljaaly, K. et al. Systematic review and meta-analysis of the safety of erythromycin compared to clarithromycin in adults and adolescents with pneumonia. J. Chemother. 32, 1–6 (2020).
Tsalik, E. L. et al. Efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections associated with low procalcitonin: a randomised, placebo-controlled, double-blind, non-inferiority trial. Lancet Infect. Dis. 23, 484–495 (2023).
Zeng, L. et al. Safety of azithromycin in pediatrics: a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 76, 1709–1721 (2020).
Fukuda, Y. et al. Efficacy and safety of macrolide therapy for adult asthma: a systematic review and meta-analysis. Respir. Investig. 62, 206–215 (2024).
Patadia, V. K. et al. Using real-world healthcare data for pharmacovigilance signal detection – the experience of the EU-ADR project. Expert Rev. Clin. Pharmacol. 8, 95–102 (2014).
Wang, Y., Zhao, B., Yang, H. & Wan, Z. A real-world pharmacovigilance study of FDA Adverse Event Reporting System events for sildenafil. Andrology 12, 785–792 (2024).
Zhao, B. et al. A real-world disproportionality analysis of Everolimus: data mining of the public version of FDA Adverse Event Reporting System. Front. Pharmacol. 15, 1333662 (2024).
Yang, H. et al. A real-world data analysis of topotecan in the FDA Adverse Event Reporting System (FAERS) database. Expert Opin. Drug Metab. Toxicol. 19, 217–223 (2023).
Inácio, P., Cavaco, A. & Airaksinen, M. The value of patient reporting to the pharmacovigilance system: a systematic review. Br. J. Clin. Pharmacol. 83, 227–246 (2017).
Sakaeda, T., Tamon, A., Kadoyama, K. & Okuno, Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int. J. Med. Sci. 10, 796–803 (2013).
Rothman, K. J., Lanes, S. & Sacks, S. T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 13, 519–523 (2004).
Brown, E. G., Wood, L. & Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20, 109–117 (1999).
Singh, S. & Loke, Y. K. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials 13, 1–8 (2012).
Kumar, A. The newly available FAERS public dashboard: implications for health care professionals. Hosp. Pharm. 54, 75–77 (2019).
Kagkelaris, K. A., Makri, O. E., Georgakopoulos, C. D. & Panayiotakopoulos, G. D. An eye for azithromycin: review of the literature. Ther. Adv. Ophthalmol. 10, 1–14 (2018).
Shaeer, K. M., Chahine, E. B., Varghese Gupta, S. & Cho, J. C. Macrolide allergic reactions. Pharmaceuticals7, 1–23 (2019).
Assa’ad, A. Three cases with three different causes of lip and mouth swelling. World Allergy Organ. J. 8, A222 (2015).
Wuollet, E., Laisi, S., Salmela, E., Ess, A. & Alaluusua, S. Molar-incisor hypomineralization and the association with childhood illnesses and antibiotics in a group of Finnish children. Acta Odontol. Scand. 74, 416–422 (2016).
National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management. NICE Guideline [NG1]. https://www.nice.org.uk/guidance/ng1 (2015).
Zimmer, V. & Emrich, K. Azithromycin-induced pill esophagitis. GE Port. J. Gastroenterol. 28, 225–226 (2021).
Camilleri, M. et al. Pharmacological, pharmacokinetic, and pharmacogenomic aspects of functional gastrointestinal disorders. Gastroenterology 150, 1319–1331 (2016).
Czarnetzki, C. et al. Erythromycin for gastric emptying in patients undergoing general anesthesia for emergency surgery: A randomized clinical trial. JAMA Surg. 150, 730–737 (2015).
van den Bosch, S., Witteman, E., Kho, Y. & Tan, A. C. Erythromycin to promote bedside placement of a self-propelled nasojejunal feeding tube in non-critically ill patients having pancreatitis: a randomized, double-blind, placebo-controlled study. Nutr. Clin. Pract. 26, 181–185 (2011).
Dirie, A. M. H., Abdinur, A. H., Osman, M. A. & Idiris, M. H. Clarithromycin induced acute pancreatitis: a rare side effect. Case report. Ann. Med. Surg.77, 103601 (2022).
Hill, K. et al. Risk of hospitalization with hemorrhage among older adults taking clarithromycin vs azithromycin and direct oral anticoagulants. JAMA Intern. Med. 180, 1052–1060 (2020).
Miyauchi, R., Kinoshita, K. & Tokuda, Y. Clarithromycin-induced haemorrhagic colitis. BMJ Case Rep. 2013, bcr2013200731 (2013).
Funding
This work was supported by the Natural Science Foundation of Hubei Province, China (Grant No. 2024AFB411); the Yichang Medical and Health Research Project (Grant No. A25-2-007); the Teaching Research Project of China Three Gorges University (Grant No. J2022075); and the Higher Education Research Project of China Three Gorges University (Grant No. GJ2342).
Author information
Authors and Affiliations
Contributions
Haiping Yao, Guoping Gan, and Zhu Wang were responsible for data collection and verification, as well as study conceptualization and design. All authors participated in data analysis and interpretation, contributed to writing the initial draft, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Gan, G. & Yao, H. Gastrointestinal safety of oral erythromycin, clarithromycin, and azithromycin in pediatric patients: a FAERS pharmacovigilance study. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04312-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04312-6
This article is cited by
-
Oral macrolide-related AEs in paediatric patients
Reactions Weekly (2025)
-
Advances in the Medical Management of Pediatric Blepharokeratoconjunctivitis
Advances in Therapy (2025)