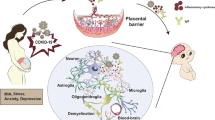
Abstract
Introduction
The impact of maternal SARS-CoV-2 infection on fetal brain development during pregnancy remains unclear. Prior research has associated other antenatal infections with adverse neurodevelopmental outcomes in offspring.
Objective
To compare neurodevelopmental outcomes in infants born to mothers infected with SARS-CoV-2 during pregnancy (COVID+) to infants without congenital exposure (COVID−).
Methods
This study included 77 COVID+ infants and 157 COVID- infants assessed at 6 and/or 12 months. Outcomes were based on maternal self-report, observed infant behavior and brain fMRI.
Results
Overall, COVID+ and COVID− infant groups showed no significant differences across a range of neurobehavioral measures. However, analyses not adjusted for multiple comparisons revealed differences: fewer night awakenings at 6 (t(154) = 2.24, p < 0.03) and 12 months (t(107) = 1.94, p < 0.05), and reduced duration of orienting at 12 months (t(55.38) = 2.15, p < 0.04) in COVID+ infants. Neural differences were noted in posterior-anterior midline, insular-frontal, insular-posterior cingulate, and frontal-cingulate regions at an uncorrected threshold of p < 0.01.
Conclusion
This study of multi-level infant development suggests that infants born to mothers infected with COVID during pregnancy are not experiencing harmful effects of that exposure.
Impact
-
This study contributes comprehensive data on infant neurodevelopmental outcomes following prenatal SARS-CoV-2 exposure, evaluating a wide range of behavioral and neural measures to address gaps in previous research.
-
Findings suggest that congenital exposure to SARS-CoV-2 does not result in significant neurodevelopmental impairments in infants, offering reassurance amidst concerns about potential long-term effects of maternal prenatal COVID-19 infection.
-
Results indicate that any observed differences, such as fewer night awakenings and functional neural connectivity patterns, may reflect a more mature developmental profile in the exposed group.
-
Continued longitudinal research is necessary to understand behaviorally relevant and lasting neurodevelopmental effects of prenatal SARS-CoV-2 exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The investigators contributing to this manuscript are committed to the principles of open and reproducible science. Data from our fetal and infant neuroimaging studies are available on OpenNeuro and on the NIMH NDAR Data Archive. Any additional queries can be directed to the corresponding author.
References
According to the World Health Organization WHO (https://covid19.who.int), as of May 12, 2025, there have been over 778 million cases of coronavirus (COVID-19) worldwide, and over 103 million in the United States. (2023).
Freedman, R. et al. Maternal choline and respiratory coronavirus effects on fetal brain development. J. Psychiatr. Res 128, 1–4 (2020).
Capretti, M. G. et al. Infants born following SARS-CoV-2 infection in pregnancy. Pediatrics 150, e2022056206 (2022).
Han, V. X., Patel, S., Jones, H. F. & Dale, R. C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 17, 564–579 (2021).
Schwartz, D. A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab Med. 144, 799–805 (2020).
Mahyuddin, A. P. et al. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2s. Prenat. Diagn. 40, 1655–1670 (2020).
Lu-Culligan, A. et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Medecine 2, 591–610 e510 (2021).
Sherer, M. L. et al. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. 225, 301 e301–301 e314 (2021).
Garcia-Flores, V. et al. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Res. Sq rs.3, rs–362886 (2021).
Edlow, A. G. et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 3, e2030455 (2020).
Gee, S. et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 22, 1490–1502 (2021).
Edlow, A. G., Castro, V. M., Shook, L. L., Kaimal, A. J. & Perlis, R. H. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw. Open 5, e2215787 (2022).
Ayed, M. et al. Neurodevelopmental outcomes of infants born to mothers with SARS-CoV-2 infections during pregnancy: a national prospective study in Kuwait. BMC Pediatr. 22, 319 (2022).
Squires, J. & Bricker, D. Ages & Stages Questionnaires, Third Edition (ASQ-3) (Paul H. Brookes Publishing, 2009).
Shuffrey, L. C. et al. Association of birth during the COVID-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr. 176, e215563 (2022).
Firestein, M. R. et al. Assessment of neurodevelopment in infants with and without exposure to asymptomatic or mild maternal SARS-CoV-2 infection during pregnancy. JAMA Netw. Open 6, e237396 (2023).
Werchan, D. M. et al. Effects of prenatal psychosocial stress and COVID-19 infection on infant attention and socioemotional development. Pediatr. Res. 95, 1279–1287 (2023).
Mappa, I. et al. Fetal brain biometry and cortical development after maternal SARS-CoV-2 infection in pregnancy: a prospective case-control study. J. Clin. Ultrasound 51, 639–643 (2023).
Kienast, P. et al. SARS-CoV-2 variant-related abnormalities detected by prenatal MRI: a prospective case-control study. Lancet Reg. Health Eur. 26, 100587 (2023).
Rizzo, G. et al. Effect of SARS-CoV-2 infection on fetal umbilical vein flow and cardiac function: a prospective study. J. Perinat. Med. 50, 398–403 (2022).
Andescavage, N. et al. Intrauterine exposure to SARS-CoV-2 infection and early newborn brain development. Cereb. Cortex 34, bhae041 (2024).
The Covid Generation (COVGEN) Research Alliance. (https://www.covgen.org/, 2020).
Thomason, M. E., Graham, A. & VanTieghem, M. R. COVID-19 and Perinatal Experiences (COPE) Vol. https://dr2.nlm.nih.gov/search/?q=21878 (U.S. National Library of Medicine, Disaster Research Response (DR2), 2020).
Thomason, M. E., Werchan, D. & Hendrix, C. L. COVID-19 patient accounts of illness severity, treatments and lasting symptoms. Sci. Data 9, 2 (2022).
Derogatis, L. R. Brief symptom inventory. Eur. J. Psychol. Assess. (1975).
Radloff, L. S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
Cox, J. L., Holden, J. M. & Sagovsky, R. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. Br. J. Psychiatry 150, 782–786 (1987).
Tronick, E., Als, H., Adamson, L., Wise, S. & Brazelton, T. B. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J. Am. Acad. Child Psychiatry 17, 1–13 (1978).
Briggs-Gowan, M. J. & Carter, A. S. BITSEA Brief Infant-Toddler Social and Emotional Assessment-Examiner’s Manual (Pearson, 2006).
Sadeh, A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 113, e570–e577 (2004).
Rothbart, M. K. Measurement of Temperament in Infancy. Child Dev. 52, 569–578 (1981).
Putnam, S. P., Helbig, A. L., Gartstein, M. A., Rothbart, M. K. & Leerkes, E. Infant behavior questionnaire--revised; very short form. J. Person. Assess. 99, 561–573 (2014).
Werchan, D. M., Thomason, M. E. & Brito, N. H. OWLET: an automated, open-source method for infant gaze tracking using smartphone and webcam recordings. Behav. Res. Methods 55, 1–15 (2022).
R Development Core Team. A language and environment for statistical computing. http://www.R-project.org (2009).
Li, Y. O., Adalı, T. & Calhoun, V. D. Estimating the number of independent components for functional magnetic resonance imaging data. Hum. Brain Mapp. 28, 1251–1266 (2007).
Himberg, J., Hyvärinen, A. & Esposito, F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22, 1214–1222 (2004).
Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141 (2012).
Chen, H., Cohen, P. & Chen, S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun. Stat. Simul. Comput. 39, 860–864 (2010).
Thomason, M. E. Standards for objectivity and reproducibility in high-impact developmental studies-the COVID-19 pandemic and beyond. JAMA Pediatr. 176, 227–228 (2022).
Rothbart, M. K., Sheese, B. E., Rueda, M. R. & Posner, M. I. Developing mechanisms of self-regulation in early life. Emot. Rev. 3, 207–213 (2011).
Komsi, N. et al. Continuity of temperament from infancy to middle childhood. Infant Behav. Dev. 29, 494–508 (2006).
Kochanska, G., Murray, K. T. & Harlan, E. T. Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Dev. Psychol. 36, 220 (2000).
Hendry, A. et al. Atypical development of attentional control associates with later adaptive functioning, autism and ADHD traits. J. Autism Dev. Disord. 50, 4085–4105 (2020).
Bruni, O. et al. Longitudinal study of sleep behavior in normal infants during the first year of life. J. Clin. Sleep. Med. 10, 1119–1127 (2014).
Krönke, K. M. et al. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia 149, 107667 (2020).
Thomason, M. E. Structured spontaneity: building circuits in the human prenatal brain. Trends Neurosci. 41, 1–3 (2018).
Acknowledgements
The authors thank Maggie Zhang, Harini Srinivasan, Amyn Majbri, Amy Hume, Leslie Gaines for contributions to data collection and data management. Importantly, the authors also thank participant families who generously shared their time and expressed interest in helping future babies to achieve their best possible health outcomes. Funding. This project was supported by awards from the National Institutes of Health, MH122447, MH125870, MH126468, DA055338, and ES032294, and by NARSAD Young Investigator Grants from the Brain and Behavior Foundation (to DMW and CM).
Author information
Authors and Affiliations
Contributions
MET and NHB designed the study and contributed materials. DW, CH, LJ, IM, BS, and MD analyzed the data. MET, DW, LJ, NHB and CM conceptualized and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors report no biomedical financial interests or potential conflicts of interest.
Consent for publication
The research protocol for this study was approved by the NYU Langone Institutional Review Board (IRB). Only patients who had previously consented to be contacted about research opportunities were eligible for invitation into the study. Participants provided consent to share de-identified data. The approved study protocol included the sharing of de-identified data with outside researchers or research databases.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomason, M.E., Werchan, D.M., Ji, L. et al. COVID-19 infection during pregnancy and infant neurodevelopment. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04409-y
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04409-y