Abstract
Background
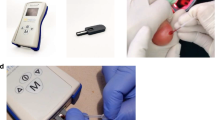
Neonatal hyperbilirubinemia is common in India, yet many public hospitals lack access to reliable and timely bilirubin testing. We evaluated the diagnostic performance of a point-of-care (POC) bilirubin device, Bilistick 2.0, in a high-volume neonatal setting.
Methods
We conducted a prospective diagnostic accuracy study at Madras Medical College, Chennai. Neonates ≥28 weeks and ≤10 days old with jaundice or at high risk were enrolled. Paired TSB samples (Bilistick and reference lab) were collected from 153 neonates. Bland-Altman analysis, correlation, and subgroup comparisons were performed.
Results
Of 153 paired samples, 137 non-hemolyzed pairs were analyzed. Mean TSB was 14.39 ± 4.03 mg/dL (Bilistick) vs. 14.27 ± 3.73 mg/dL (reference). Mean absolute difference was 1.35 mg/dL; 79% of values were within ±2 mg/dL. Mean bias was +0.13 mg/dL. Correlation was r = 0.88 overall, and r = 0.96 for TSB > 20 mg/dL. Test failure rate was 10.5%, mostly during the initial learning phase.
Conclusion
Bilistick 2.0 showed strong diagnostic agreement with reference laboratory bilirubin testing. With training and basic laboratory coordination, it may support safe, rapid bilirubin management in resource-limited neonatal care.
Impact
-
The Bilistick 2.0 point-of-care device showed strong diagnostic agreement with reference laboratory bilirubin testing.
-
Performance remained reliable in neonates with clinically significant bilirubin levels, especially >20 mg/dL.
-
Turnaround time was under 15 min, and user-related test failures decreased after brief training.
-
The device has potential for decentralized bilirubin monitoring in public-sector neonatal care in India.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policy and participant confidentiality, but are available from the corresponding author on reasonable request.
References
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Kemper, A. R. et al. Clinical Practice Guideline Revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 150, e2022058859 (2022).
National Collaborating Centre for Women’s and Children’s Health. Neonatal jaundice. http://guidance.nice.org.uk/CG98 (NICE, 2010).
Bhutani, V. K. et al. Neonatal hyperbilirubinemia, and Rhesus disease of the newborn: incidence and impairment estimate for 2010 at regional and global levels. Pediatr. Res. 74, 86–100 (2013).
Vidavalur, R. & Bhutani, V. K. Managing the Historic Burden of Kernicterus Mortality in India. Indian J. Pediatr. 91, 1262–1267 (2024).
Hulzebos, C. V. et al. Bilirubin measurements in neonates: uniform neonatal treatment can only be achieved by improved standardization. Clin. Chem. Lab. Med. 62, 1892–1903 (2024).
Lo, S. F. Commentary on clinicians at crossroads for a dangerous interference in neonatal Bilirubin determination at the point-of-care. Clin. Chem. 68, 892 (2022).
Lo, S. F. & Doumas, B. T. The status of bilirubin measurements in U.S. laboratories: Why is accuracy elusive?. Semin. Perinatol. 35, 141–147 (2011).
Bilistick System of Bilimetrix, Inc. https://www.bilimetrix.net/wp-content/themes/hypnotherapy-child/pdf/Bilistick_System%20Technical_DataSheet_EN.pdf.
Lee, A. C. et al. A novel icterometer for hyperbilirubinemia screening in low-resource settings. Pediatrics 143, e20182039 (2019).
Okwundu, C. I. et al. Transcutaneous bilirubinometry for detecting jaundice in term or late preterm neonates. Cochrane Database Syst. Rev. 5, CD011060 (2024).
Westenberg, L. E. H. et al. Diagnostic accuracy of portable, handheld point-of-care tests vs laboratory-based bilirubin quantification in neonates. JAMA Pediatr. 177, 479–479 (2023).
Zabetta, C. D. et al. A low-cost point-of-care system to measure total plasma Bilirubin. Neonatology 103, 177–181 (2013).
Maisels, M. J. et al. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics 124, 1193–1198 (2009).
Boo, N. Y. et al. The point-of-care Bilistick method has very short turn-around-time and high accuracy at lower cutoff levels to predict laboratory-measured TSB. Pediatr. Res. 86, 216–220 (2019).
Greco, C. et al. Diagnostic performance analysis of the point-of-care bilistick system in identifying severe neonatal hyperbilirubinemia by a multi-country approach. EClinicalMedicine 1, 14–20 (2018).
Thielemans, L. et al. Laboratory validation and field usability assessment of a point-of-care test for serum bilirubin levels in neonates in a tropical setting. Wellcome Open Res. 3, 110 (2018).
Greco, C. et al. Comparison between Bilistick System and transcutaneous bilirubin in assessing total bilirubin serum concentration in jaundiced newborns. J. Perinatol. 37, 1028–1031 (2017).
Sampurna, M. T. A. et al. Diagnostic properties of a portable point-of-care method to measure bilirubin and a transcutaneous bilirubinometer. Neonatology 118, 678–684 (2021).
Mather, A. Reliability of bilirubin determinations in icterus of the newborn infant. Pediatrics 26, 350–354 (1960).
American Academy of Pediatrics, College of American Pathologists, American Association for Clinical Chemistry, National Institutes of Health Uniformity of bilirubin standards. Clin. Chem. 8, 405–406 (1962).
National Institute of Standards and Technology. Certificate of Analysis: Standard Reference Material 916a, Bilirubin. (NIST, 2001).
Doumas, B. T. et al. Candidate reference method for determination of total bilirubin in serum: development and validation. Clin. Chem. 31, 1779–1789 (1985).
Acknowledgements
The authors thank Professor Vinod K. Bhutani and Professor Claudio Tiribelli for their expert guidance throughout the study. The authors also acknowledge Mr. Sashi Kumar of Phoenix Medical Systems, and the team at Bilimetrix, for their support in providing the Bilistick 2.0 devices and consumables required for this research. The authors gratefully acknowledge the Government of India, Ministry of Health & Family Welfare, Department of Health Research, Multidisciplinary Research Unit (MRU), and the infrastructural facility of Madras Medical College.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: Mangalabharathi Sundaram, Arunkumar Muthusamy, Anitha Balachandran, Muthukumaran Natarajan. Drafting the article or revising it critically for important intellectual content: Mangalabharathi Sundaram, Arunkumar Muthusamy, Anitha Balachandran, Muthukumaran Natarajan. Final approval of the version to be published: Mangalabharathi Sundaram, Arunkumar Muthusamy, Anitha Balachandran, Muthukumaran Natarajan.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from the parents or legal guardians of all study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sundaram, M., Muthusamy, A., Balachandran, A. et al. Diagnostic performance of point-of-care bilirubin testing with Bilistick 2.0 device at a South Indian clinical site. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04431-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04431-0
This article is cited by
-
Need for accurate and actionable neonatal bilirubin test results
Pediatric Research (2025)