Abstract
Background
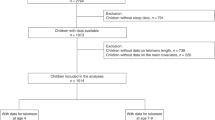
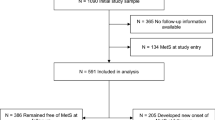
Evidence on the link between physical activity (PA) and telomere length (TL) in childhood is scarce and inconsistent. This study examined the association between extracurricular PA at age 4 and changes in TL ranking from 4 to 8 years.
Methods
Longitudinal data from 547 children in the INMA birth cohort study (ages 4–8) were analyzed. Parent-reported extracurricular PA at age 4 was used to calculate metabolic equivalents (METs) in hours per day and categorized into tertiles (low, middle, and high). Leukocyte TL was measured at ages 4 and 8 using qPCR, with the primary outcome being the percentage change in TL ranking between ages 4 and 8. Multiple robust regression models were used for the main analyses.
Results
Children in the highest tertile of extracurricular PA (11.9–31.0 METs h/day) showed a significant 2.25% increase (95% CI: 0.01, 4.48; p = 0.04) in TL ranking between 4 and 8 years compared to the lowest tertile (2.2–7.8 METs h/day). No association was observed for moderate extracurricular PA (i.e., middle tertile) levels.
Conclusions
Higher levels of extracurricular PA were prospectively associated with TL rank changes from 4 to 8 years, suggesting its potential to reduce cellular damage and support healthy ageing.
Impact
-
Research shows an association between PA and TL maintenance in adults, but evidence in childhood is limited and inconsistent.
-
This study investigates the association between extracurricular PA and changes in TL ranking in children aged 4–8 years, finding that higher PA levels are linked to increased TL ranking, possibly slowing telomere shortening.
-
Findings support promoting PA in childhood to enhance cellular health and reduce chronic disease risk.
-
Results can inform strategies by healthcare professionals, educators, and policymakers to encourage PA in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Researchers interested in accessing this data may submit a formal request to the corresponding author. All such requests will be thoroughly reviewed by the research team, and a data transfer agreement will be required prior to any data sharing.
References
Blackburn, E. H. Structure and function of telomeres. Nature 350, 569–573 (1991).
O’Sullivan, R. J. & Karlseder, J. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11, 171–181 (2010).
Shammas, M. A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 14, 28–34 (2011).
Wang, Q., Zhan, Y., Pedersen, N. L., Fang, F. & Hägg, S. Telomere length and all-cause mortality: a meta-analysis. Ageing Res. Rev. 48, 11–20 (2018).
Fyhrquist, F., Saijonmaa, O. & Strandberg, T. The roles of senescence and telomere shortening in cardiovascular disease. Nat. Rev. Cardiol. 10, 274–283 (2013).
Dugdale, H. L. & Richardson, D. S. Heritability of telomere variation: it is all about the environment! Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160450 (2018).
Reichert, S. & Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463 (2017).
Kordinas, V., Ioannidis, A. & Chatzipanagiotou, S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect? Genes 7, 60 (2016).
Zong, Z.-Q. et al. Ambient air pollution exposure and telomere length: a systematic review and meta-analysis. Public Health 215, 42–55 (2023).
Osorio-Yáñez, C. et al. Early life tobacco exposure and children’s telomere length: the HELIX project. Sci. Total Environ. 711, 135028 (2020).
Valera-Gran, D. et al. The impact of foods, nutrients, or dietary patterns on telomere length in childhood and adolescence: a systematic review. Nutrients 14, 3885 (2022).
Bountziouka, V. et al. Modifiable traits, healthy behaviours, and leukocyte telomere length: a population-based study in UK Biobank. Lancet Healthy Longev. 3, e321–e331 (2022).
Semeraro, M. D. et al. Physical activity, a modulator of aging through effects on telomere biology. Aging 12, 13803–13823 (2020).
Rebelo-Marques, A. et al. Aging hallmarks: the benefits of physical exercise. Front. Endocrinol. 9, 258 (2018).
Daskalopoulou, C. et al. Physical activity and healthy ageing: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 38, 6–17 (2017).
Garatachea, N. et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 18, 57–89 (2015).
Watts, E. L. et al. Association of leisure time physical activity types and risks of all-cause, cardiovascular, and cancer mortality among older adults. JAMA Netw. Open 5, e2228510 (2022).
Arsenis, N. C., You, T., Ogawa, E. F., Tinsley, G. M. & Zuo, L. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget 8, 45008–45019 (2017).
Schellnegger, M., Lin, A. C., Hammer, N. & Kamolz, L.-P. Physical activity on telomere length as a biomarker for aging: a systematic review. Sports Med. Open 8, 111 (2022).
Santovito, A., Agostinovna Nigretti, A., Sellitri, A., Scarfò, M. & Nota, A. Regular sport activity is able to reduce the level of genomic damage. Biology 12, 1110 (2023).
Aubert, G., Baerlocher, G. M., Vulto, I., Poon, S. S. & Lansdorp, P. M. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 8, e1002696 (2012).
Guxens, M. et al. Cohort profile: the INMA-INfancia y Medio Ambiente-(Environment and Childhood) Project. Int. J. Epidemiol. 41, 930–940 (2012).
Ridley, K., Ainsworth, B. E. & Olds, T. S. Development of a compendium of energy expenditures for youth. Int. J. Behav. Nutr. Phys. Act. 5, 45 (2008).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 (2007).
Telomere Research Network. Study design & analysis. https://trn.tulane.edu/resources/study-design-analysis/ (2023).
Notario-Barandiaran, L. et al. High adherence to a Mediterranean diet at age 4 reduces overweight, obesity and abdominal obesity incidence in children at the age of 8. Int. J. Obes. 44, 1906–1917 (2020).
Monteiro, C. A. et al. NOVA. The star shines bright. World Nutr. 7, 28–38 (2016).
Vioque, J. et al. Reproducibility and validity of a food frequency questionnaire designed to assess diet in children aged 4-5 years. PLoS ONE 11, e0167338 (2016).
Maechler, M. et al. robustbase: Basic robust statistics (CRAN, 2021).
Bijnens, E. M. et al. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med. 15, 205 (2017).
Balduzzi, S., Rücker, G. & Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. Evid. Based Ment. Health 22, 153–160 (2019).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Canudas, S. et al. Mediterranean diet and telomere length: a systematic review and meta-analysis. Adv. Nutr. 11, 1544–1554 (2020).
Petermann-Rocha, F. et al. Children who sleep more may have longer telomeres: evidence from a longitudinal population study in Spain. Pediatr. Res. 93, 1419–1424 (2023).
Martens, D. S., Plusquin, M., Gyselaers, W., De Vivo, I. & Nawrot, T. S. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 14, 148 (2016).
Belfort, M. B. et al. Telomere length shortening in hospitalized preterm infants: a pilot study. PLoS ONE 16, e0243468 (2021).
De Ruyter, T. et al. A multi-exposure approach to study telomere dynamics in childhood: a role for residential green space and waist circumference. Environ. Res. 213, 113656 (2022).
Zhu, H. et al. Leukocyte telomere length in healthy White and Black adolescents: relations to race, sex, adiposity, adipokines and physical activity. J. Pediatr. 158, 215–220 (2011).
García-Calzón, S. et al. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: the EVASYON study. PLoS ONE 9, e89828 (2014).
Ojeda-Rodríguez, A. et al. Association between favourable changes in objectively measured physical activity and telomere length after a lifestyle intervention in pediatric patients with abdominal obesity. Appl. Physiol. Nutr. Metab. 46, 205–212 (2021).
Paltoglou, G. et al. A comprehensive, multidisciplinary, personalized, lifestyle intervention program is associated with increased leukocyte telomere length in children and adolescents with overweight and obesity. Nutrients 13, 2682 (2021).
Delisle Nyström, C., Migueles, J. H., Henriksson, P. & Löf, M. Physical activity and cardiovascular risk factors in children from 4 to 9 years of age. Sports Med. Open 9, 99 (2023).
García-Hermoso, A., Ezzatvar, Y., Ramírez-Vélez, R., Olloquequi, J. & Izquierdo, M. Is device-measured vigorous physical activity associated with health-related outcomes in children and adolescents? A systematic review and meta-analysis. J. Sport Health Sci. 10, 296–307 (2021).
Migueles, J. H., Delisle Nyström, C., Leppänen, M. H., Henriksson, P. & Löf, M. Revisiting the cross-sectional and prospective association of physical activity with body composition and physical fitness in preschoolers: a compositional data approach. Pediatr. Obes. 17, e12909 (2022).
Reisberg, K., Riso, E.-M. & Jürimäe, J. Associations between physical activity, body composition, and physical fitness in the transition from preschool to school. Scand. J. Med. Sci. Sports 30, 2251–2263 (2020).
Ried-Larsen, M. et al. Associations between objectively measured physical activity intensity in childhood and measures of subclinical cardiovascular disease in adolescence: prospective observations from the European Youth Heart Study. Br. J. Sports Med. 48, 1502–1507 (2014).
Väistö, J. et al. Longitudinal associations of physical activity and sedentary time with cardiometabolic risk factors in children. Scand. J. Med. Sci. Sports 29, 113–123 (2019).
Ortega, F. B., Ruiz, J. R., Castillo, M. J. & Sjöström, M. Physical fitness in childhood and adolescence: a powerful marker of health. Int. J. Obes. 32, 1–11 (2008).
Skrede, T., Steene-Johannessen, J., Anderssen, S. A., Resaland, G. K. & Ekelund, U. The prospective association between objectively measured sedentary time, moderate-to-vigorous physical activity and cardiometabolic risk factors in youth: a systematic review and meta-analysis. Obes. Rev. 20, 55–74 (2019).
Chaput, J.-P. et al. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5-17 years: summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 17, 141 (2020).
Kim, J. J., Ahn, A., Ying, J., Hickman, E. & Ludlow, A. T. Exercise as a therapy to maintain telomere function and prevent cellular senescence. Exerc. Sport Sci. Rev. 51, 150–160 (2023).
Mundstock, E. et al. Effects of physical activity in telomere length: systematic review and meta-analysis. Ageing Res. Rev. 22, 72–80 (2015).
Sellami, M., Bragazzi, N., Prince, M. S., Denham, J. & Elrayess, M. Regular, intense exercise training as a healthy aging lifestyle strategy: preventing DNA damage, telomere shortening and adverse DNA methylation changes over a lifetime. Front. Genet. 12, 652497 (2021).
Sarker, H. et al. Validation of parent-reported physical activity and sedentary time by accelerometry in young children. BMC Res. Notes 8, 735 (2015).
Telama, R. et al. Tracking of physical activity from early childhood through youth into adulthood. Med. Sci. Sports Exerc. 46, 955–962 (2014).
García-Hermoso, A., Izquierdo, M. & Ramírez-Vélez, R. Tracking of physical fitness levels from childhood and adolescence to adulthood: a systematic review and meta-analysis. Transl. Pediatr. 11, 386–401 (2022).
Funding
This research was funded by Instituto de Salud Carlos III/Agencia Estatal de Investigación, grant number PI18/00825 Project: “Dieta y actividad física en embarazo y tras el nacimiento y longitud del telómero en niños y adolescentes: Proyecto TeloDiPA” and Unión Europea (FEDER) “Una manera de hacer Europa”; grant number PI15/00118: “Exposiciones ambientales a edad temprana y riesgo cardiovascular del adolescente (INMA-Cardio); and Generalitat Valenciana (GVA/2021/191). ISGlobal acknowledges support from the grant CEX2018-000806-S funded by MCIN/AEI/10.13039/501100011033, and support from the Generalitat de Catalunya through the CERCA Program.
Author information
Authors and Affiliations
Contributions
This study was conceptualized and designed by E.-M.N.-M. and D.V.-G. D.P.-B., D.S.M., and T.N. performed the acquisition, analysis, and interpretation of data. The formal statistical analysis was conducted by D.P.-B. and E.-M.N.-M. D.V.-G. and D.P.-B. prepared the first manuscript draft. Following drafts and the final manuscript version were written by D.V.-G. All authors critically reviewed the final manuscript and approved its submission to the journal. E.-M.N.-M. and D.V.-G. supervised the development of the study. E.-M.N.-M. is the guarantor.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of General Hospital of the Department of Health of Alicante (protocol code Acta 2019/07 and date of approval on 31st July 2019). All parents/legal tutors provided their written consent at each phase of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valera-Gran, D., Prieto-Botella, D., Martens, D.S. et al. Extracurricular physical activity and telomere length in childhood: findings from the INMA study. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04445-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04445-8
This article is cited by
-
Commentary: Extracurricular physical activity as an early biological investment
Pediatric Research (2026)