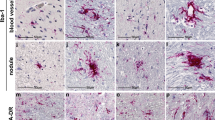
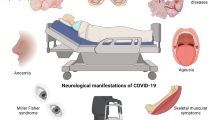
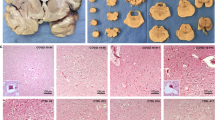
Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been shown to cause a unique disease phenotype in the paediatric population compared to adults, following the emergence of Multisystem Inflammatory Syndrome of Children (MIS-C) and Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS). Over the course of the pandemic, neurological symptoms associated with SARS-CoV-2 have been reported in the paediatric population. The neurological and neurodevelopmental sequelae of both acute SARS-CoV-2 infection and MIS-C/PIMS-TS in the paediatric population are not well understood. Little is known about the underlying pathophysiology and the potential neurovirulence of SARS-CoV-2. Further awareness and research are needed on the neurological sequelae and long-term consequences of SARS-CoV-2 on the developing brain.
Impact
-
Detailed review of the current knowledge on neurological and neurodevelopmental effects of SARS-CoV-2 and MIS-C on the paediatric population.
-
Emphasise the importance of acknowledging the potential direct effects of SARS-CoV-2 and MIS-C on neurological and neurodevelopmental outcomes.
-
Highlight the need for further research and inclusion of paediatric patients in follow up studies of long-term effects of SARS-CoV-2 and MIS-C focusing on neurodevelopmental and neurological sequelae.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Centers for Disease Control and Prevention. Covid Data Tracker, <https://covid.cdc.gov/covid-data-tracker> (2023).
Centers for Disease Control and Prevention. Information for Pediatric Healthcare Providers, <https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatric-hcp.html> (2023).
Royal College of Paediatrics and Child Health. Covid-10 Research Evidence Summaries, <https://www.rcpch.ac.uk/resources/COVID-19-research-evidence-summaries> (2021).
Tsankov, B. K. et al. Severe Covid-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J. Infect. Dis. 103, 246–256 (2021).
Riphagen, S., Gomez, X., Gonzalez-Martinez, C., Wilkinson, N. & Theocharis, P. Hyperinflammatory shock in children during Covid-19 pandemic. Lancet 395, 1607–1608 (2020).
Pawar, R. et al. Neonatal Multisystem Inflammatory Syndrome (Mis-N) associated with prenatal maternal Sars-Cov-2: a Case Series. Children 8 572 (2021).
Nakra, N. A., Blumberg, D. A., Herrera-Guerra, A. & Lakshminrusimha, S. Multi-system inflammatory syndrome in Children (Mis-C) following Sars-Cov-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children 7 69 (2020).
Santos, M. O. et al. Multisystem inflammatory syndrome (Mis-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J. Pediatr. 98, 338–349 (2022).
Centers for Disease Control and Prevention. Standardized Case Definition for Surveillance of Multisystem Inflammatory Syndrome in Children Associated with Sars-Cov-2 Infection, <https://www.cdc.gov/mis-c/hcp_cstecdc/index.html> (2023).
World Health Organization. A Clinical Case Defintion for Post Covid-10 Condition in Children and Adolescence by Expert Consensus, <https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-Covid-19-condition-CA-Clinical-case-defintion-2023-1> (2023).
Feldstein, L. R. et al. Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (Mis-C) compared with severe acute Covid-19. JAMA 325, 1074–1087 (2021).
Hoste, L., Van Paemel, R. & Haerynck, F. Multisystem inflammatory syndrome in children related to Covid-19: a systematic review. Eur. J. Pediatr. 180, 2019–2034 (2021).
Abdel-Mannan, O. et al. Neurologic and radiographic findings associated with Covid-19 infection in children. JAMA Neurol. 77, 1440–1445 (2020).
Antoon, J. W. et al. Covid-19 and acute neurologic complications in children. Pediatrics 150 69 (2022).
Stafstrom, C. E. Neurological effects of Covid-19 in infants and children. Dev. Med. Child Neurol. 64, 818–829 (2022).
Mulkey, S. B., Bearer, C. F. & Molloy, E. J. Indirect effects of the Covid-19 pandemic on children relate to the child’s age and experience. Pediatr. Res 94, 1586–1587 (2023).
Bauer, L. et al. The neuroinvasiveness, neurotropism, and neurovirulence of Sars-Cov-2. Trends Neurosci. 45, 358–368 (2022).
Roya College of Paediatrics and Child Health. Paediatric Multisystem Inflammatory Syndrome Temporally Associated with Covid-19 (PIMS)- Guidance for Clinicians, <https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance> (2020).
Molloy, E. J., Nakra, N., Gale, C., Dimitriades, V. R. & Lakshminrusimha, S. Multisystem Inflammatory Syndrome in Children (Mis-C) and Neonates (Mis-N) associated with Covid-19: optimizing definition and management. Pediatr. Res 93, 1499–1508 (2023).
Zambrano, L. D. et al. Risk Factors for multisystem inflammatory syndrome in children: a case-control investigation. Pediatr. Infect. Dis. J. 42, e190–e196 (2023).
Lakshminrusimha, S., Hudak, M. L., Dimitriades, V. R. & Higgins, R. D. Multisystem inflammatory syndrome in neonates following maternal Sars-Cov-2 Covid-19 infection. Am. J. Perinatol. 39, 1166–1171 (2022).
Singer, T. G., Evankovich, K. D., Fisher, K., Demmler-Harrison, G. J. & Risen, S. R. Coronavirus Infections in the nervous system of children: a scoping review making the case for long-term neurodevelopmental surveillance. Pediatr. Neurol. 117, 47–63 (2021).
Jamali, Z., Sinaei, R. & Razi, L. Multisystem Inflammatory Syndrome in a Newborn (Mis-N): clinical evidence and neurodevelopmental outcome. Curr. Pediatr. Rev. 19, 210–212 (2023).
Fink, E. L. et al. Prevalence and risk factors of neurologic manifestations in hospitalized children diagnosed with acute Sars-Cov-2 or Mis-C. Pediatr. Neurol. 128, 33–44 (2022).
Francoeur, C. et al. Severe pediatric neurological manifestations with Sars-Cov-2 or Mis-C hospitalization and new morbidity. JAMA Netw. Open 7, e2414122 (2024).
Lee, I. T., Lin, P. J. & Yen, H. H. Pediatric neuroimaging findings and clinical presentations of Covid-19: a systematic review. Int. J. Infect. Dis. 138, 29–37 (2024).
Kwan, A. T. H. et al. Association of Sars-Cov-2 infection with neurological symptoms and neuroimaging manifestations in the pediatric population: a systematic review. J. Psychiatr. Res. 170, 90–110 (2024).
Sakuma, H. et al. International consensus definitions for infection-triggered encephalopathy syndromes. Dev. Med. Child Neurol. 67, 195–207 (2025).
Kasai, M. et al. Clinical characteristics of Sars-Cov-2-associated encephalopathy in children: nationwide epidemiological study. J. Neurol. Sci. 457, 122867 (2024).
Ma, Y. et al. Acute necrotizing encephalopathy infected with the Sars-Cov-2 in children: case series and literature review of clinical outcomes with the use of tocilizumab. Eur. J. Paediatr. Neurol. 52, 67–75 (2024).
Helbok, R. et al. Neurocovid: it’s time to join forces globally. Lancet Neurol. 19, 805–806 (2020).
Lindan, C. E. et al. Neuroimaging manifestations in children with Sars-Cov-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc. Health 5, 167–177 (2021).
Wong, A. M. & Toh, C. H. Spectrum of neuroimaging mimics in children with Covid-19 infection. Biomed. J. 45, 50–62 (2022).
Ucan, B. et al. Multisystem inflammatory syndrome in children associated with Sars-Cov-2: extracardiac radiological findings. Br. J. Radiol. 95, 20210570 (2022).
Guerrero, J. I. et al. Central and peripheral nervous system involvement by Covid-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect. Dis. 21, 515 (2021).
Gulko, E. et al. MRI brain findings in 126 patients with Covid-19: initial observations from a descriptive literature review. Am. J. Neuroradiol. 41, 2199–2203 (2020).
Choi, Y. & Lee, M. K. Neuroimaging findings of brain MRI and CT in patients with Covid-19: a systematic review and meta-analysis. Eur. J. Radiol. 133, 109393 (2020).
LaRovere, K. L. et al. Neurologic involvement in children and adolescents hospitalized in the United States for Covid-19 or multisystem inflammatory syndrome. JAMA Neurol. 78, 536–547 (2021).
LaRovere, K. L. et al. Changes in distribution of severe neurologic involvement in US pediatric inpatients with Covid-19 or multisystem inflammatory syndrome in children in 2021 Vs 2020. JAMA Neurol. 80, 91–98 (2023).
Kremer, S. et al. Brain MRI findings in severe Covid-19: a retrospective observational study. Radiology 297, E242–E251 (2020).
Molloy, E. J. & Bearer, C. F. Covid-19 in children and altered inflammatory responses. Pediatr. Res. 88, 340–341 (2020).
Rastogi, S., Gala, F., Kulkarni, S. & Gavali, V. Neurological and neuroradiological patterns with Covid-19 infection in children: a single institutional study. Indian J. Radiol. Imaging 32, 510–522 (2022).
Sa, M. et al. Systemic inflammation is associated with neurologic involvement in pediatric inflammatory multisystem syndrome associated with SARS-Cov-2. Neurol Neuroimmunol. Neuroinflammation 8 e1023 (2021).
Jiang, N. M., Cowan, M., Moonah, S. N. & Petri, W. A. The impact of systemic inflammation on neurodevelopment. Trends Mol. Med. 24, 794–804 (2018).
DeBiasi, R. L. et al. Multisystem inflammatory syndrome of children: subphenotypes, risk factors, biomarkers, cytokine profiles, and viral sequencing. J. Pediatr. 237, 125–135.e118 (2021).
Godfred-Cato, S. et al. Distinguishing multisystem inflammatory syndrome in children from Covid-19, Kawasaki Disease and Toxic Shock Syndrome. Pediatr. Infect. Dis. J. 41, 315–323 (2022).
Morrow, A. K. et al. Postacute/long covid in pediatrics: development of a multidisciplinary rehabilitation clinic and preliminary case series. Am. J. Phys. Med Rehabil. 100, 1140–1147 (2021).
Izquierdo-Pujol, J. et al. Post Covid-19 condition in children and adolescents: an emerging problem. Front. Pediatr. 10, 894204 (2022).
Landry, M. et al. Postacute sequelae of Sars-Cov-2 in university setting. Emerg. Infect. Dis. 29, 519–527 (2023).
Lopez-Leon, S. et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci. Rep. 12, 9950 (2022).
Morrow, A. K. et al. Long-term COVID-19 sequelae in adolescents: the overlap with orthostatic intolerance and Me/Cfs. Curr. Pediatr. Rep. 10, 31–44 (2022).
Stephenson, T. et al. Physical and mental health 3 months after Sars-CoV-2 infection (Long Covid) among adolescents in England (Clock): a national matched cohort study. Lancet Child Adolesc. Health 6, 230–239 (2022).
Avittan, H. & Kustovs, D. Cognition and mental health in pediatric patients following Covid-19. Int. J. Environ. Res. Public Health 20 5061 (2023).
Morand, A. et al. Similar patterns of Eur. J. Nucl. Med. Mol. Imaging 49, 913–920 (2022).
Churchill, N. W. et al. Effects of post-acute Covid-19 syndrome on the functional brain networks of non-hospitalized individuals. Front. Neurol. 14, 1136408 (2023).
Monje, M. & Iwasaki, A. The neurobiology of long Covid. Neuron 110, 3484–3496 (2022).
Davies, S. C., Walsh-Messinger, J. & Greenspan, N. Supporting Students with Post-Acute Sequelae of SARS-Cov-2 Infection: Applying Lessons Learned from Postconcussion Symptoms Vol. 50 (National Association of School Psychologists Communique, 2021).
Keenan, H. T., Hooper, S. R., Wetherington, C. E., Nocera, M. & Runyan, D. K. Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics 119, e616–623 (2007).
Morissette, M. P., Prior, H. J., Tate, R. B., Wade, J. & Leiter, J. R. S. Associations between concussion and risk of diagnosis of psychological and neurological disorders: a retrospective population-based cohort study. Fam. Med. Community Health 8 e000390 (2020).
Taquet, M. et al. Neurological and psychiatric risk trajectories after Sars-Cov-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 9, 815–827 (2022).
Morello, R. et al. Risk factors for post-Covid-19 condition (long Covid) in children: a prospective cohort study. EClinicalMedicine 59, 101961 (2023).
Knight, M. et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed Sars-Cov-2 infection in UK: national population based cohort study. BMJ 369, m2107 (2020).
Rodriguez, V. J. et al. Prevalence of neurodevelopmental delays in infants with perinatal HIV infection in comparison with HIV exposure in Rural South Africa. AIDS (2023).
Almond, D. & Mazumder, B. The 1918 influenza pandemic and subsequent health outcomes: an analysis of Sipp data. Am. Econ. Rev. 95, 258–262 (2005).
Patterson, P. H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 (2009).
Otero, A. M. & Antonson, A. M. At the crux of maternal immune activation: viruses, microglia, microbes, and Il-17a. Immunol. Rev. 311, 205–223 (2022).
Cordeiro, C. N., Tsimis, M. & Burd, I. Infections and brain development. Obstet. Gynecol. Surv. 70, 644–655 (2015).
Al-Haddad, B. J. S. et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 76, 594–602 (2019).
Shook, L. L., Sullivan, E. L., Lo, J. O., Perlis, R. H. & Edlow, A. G. Covid-19 in pregnancy: implications for fetal brain development. Trends Mol. Med. 28, 319–330 (2022).
Woods, R. M. et al. Maternal immune activation and role of placenta in the prenatal programming of neurodevelopmental disorders. Neuronal Signal 7, NS20220064 (2023).
Shuffrey, L. C. et al. Association of birth during the Covid-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal Sars-Cov-2 infection. JAMA Pediatr. 176, e215563 (2022).
Cheng, Y. et al. Impact of Sars-Cov-2 Infection during pregnancy on infant neurobehavioral development: a case-control study. Front. Pediatr. 9, 762684 (2021).
Wu, T. et al. Effects of Sars-Cov-2 Infection during late pregnancy on early childhood development: a prospective cohort study. Front. Pediatr. 9, 750012 (2021).
Ayed, M. et al. Neurodevelopmental outcomes of infants born to mothers with Sars-Cov-2 infections during pregnancy: a national prospective study in Kuwait. BMC Pediatr. 22, 319 (2022).
Hessami, K. et al. Covid-19 pandemic and infant neurodevelopmental impairment: a systematic review and meta-analysis. JAMA Netw. Open 5, e2238941 (2022).
Ayesa-Arriola, R. et al. Exploring the impact of Covid-19 on newborn neurodevelopment: a pilot study. Sci. Rep. 13, 2983 (2023).
Aldrete-Cortez, V. et al. Infants prenatally exposed to Sars-Cov-2 show the absence of fidgety movements and are at higher risk for neurological disorders: a comparative study. PLoS One 17, e0267575 (2022).
Columbia University Irving Medical Center. The Combo Study (Covid-19 Mother Baby Outcomes Study) Initiative. <https://www.ps.columbia.edu/COMBO> (2023).
Firestein, M. R. et al. Assessment of neurodevelopment in infants with and without exposure to asymptomatic or mild maternal Sars-Cov-2 infection during pregnancy. JAMA Netw. Open 6, e237396 (2023).
Jackson, R. et al. Association of antenatal or neonatal Sars-Cov-2 exposure with developmental and respiratory outcomes, and healthcare usage in early childhood: a national prospective cohort study. EClinicalMedicine 72, 102628 (2024).
Firestein, M. R. et al. Positive autism screening rates in toddlers born during the Covid-19 pandemic. JAMA Netw. Open 7, e2435005 (2024).
Huang, P. et al. Association between the Covid-19 pandemic and infant neurodevelopment: a comparison before and during Covid-19. Front. Pediatr. 9, 662165 (2021).
Bianco, C. et al. Pandemic beyond the virus: maternal Covid-related postnatal stress is associated with infant temperament. Pediatr. Res. 93, 253–259 (2023).
Firestein, M. R., Dumitriu, D., Marsh, R. & Monk, C. Maternal mental health and infant development during the Covid-19 pandemic. JAMA Psychiatry 79, 1040–1045 (2022).
Kyle, M. H. & Dumitriu, D. The effect of Coronavirus Disease 2019 on newborns. Curr. Opin. Pediatr. 33, 618–624 (2021).
Kyle, M. H. et al. Vertical transmission and neonatal outcomes following maternal Sars-Cov-2 infection during pregnancy. Clin. Obstet. Gynecol. 65, 195–202 (2022).
Lavallee, A. & Dumitriu, D. Low risk of neurodevelopmental impairment in the Covid-19 generation should not make researchers complacent. JAMA Netw. Open 5, e2238958 (2022).
Gale, C. et al. Characteristics and outcomes of neonatal Sars-Cov-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc. Health 5, 113–121 (2021).
Ali, S. et al. Neonatal outcomes of maternal Sars-Cov-2 infection in the UK: a prospective cohort study using active surveillance. Pediatr. Res. 94, 1203–1208 (2023).
Sturrock, S., Ali, S., Gale, C., Battersby, C. & Le Doare, K. Neonatal outcomes and indirect consequences following maternal Sars-Cov-2 infection in pregnancy: a systematic review. BMJ Open 13, e063052 (2023).
Marlow, N. et al. No change in neurodevelopment at 11 years after extremely preterm birth. Arch. Dis. Child Fetal Neonatal Ed. 106, 418–424 (2021).
Yan, K. et al. Effects of Sars-Cov-2 infection on neuroimaging and neurobehavior in neonates. World J. Pediatr. 17, 171–179 (2021).
Goyal, M., Mascarenhas, D., Rr, P. & Nanavati, R. Long-term growth and neurodevelopmental outcomes of neonates infected with Sars-Cov-2 during the Covid-19 pandemic at 18–24 months corrected age: a prospective observational study. Neonatology 121, 450–459 (2024).
Yangin Ergon, E. et al. The long-term neurodevelopmental outcomes of toddlers with Sars-Cov-2 infection in the neonatal period: a prospective observational study. Ital. J. Pediatr. 50, 34 (2024).
Woodward, K. et al. Effect of Sars-Cov-2 infection in neonates or in pregnancy on developmental outcomes at 21–24 months (Sinepost): study protocol for a prospective cohort study. BMJ Paediatr. Open 6 e001571 (2022).
Gomes, I. et al. Sars-Cov-2 infection of the central nervous system in a 14-month-old child: a case report of a complete autopsy. Lancet Reg. Health Am. 2, 100046 (2021).
Casabianca, M., Caula, C., Titomanlio, L. & Lenglart, L. Neurological consequences of Sars-Cov-2 infections in the pediatric population. Front Pediatr. 11, 1123348 (2023).
Ravichandran, S. et al. Sars-Cov-2 immune repertoire in Mis-C and pediatric Covid-19. Nat. Immunol. 22, 1452–1464 (2021).
Isaza-Correa, J. et al. Innate immune dysregulation in multisystem inflammatory syndrome in children (Mis-C). Sci. Rep. 13, 16463 (2023).
Lin, J. et al. Emerging insights into the pathophysiology of multisystem inflammatory syndrome associated with Covid-19 in children. Can. J. Cardiol. 39, 793–802 (2023).
McKenna, E. et al. Neutrophils in Covid-19: not innocent bystanders. Front. Immunol. 13, 864387 (2022).
University of Oxford & Nuffield Department of Medicine. Recovery trial (Randomised Evaluation of Covid-19 Therapy), <https://www.recoverytrial.net>.
Welzel, T. et al. Multicenter randomized trial of methylprednisolone vs. intravenous immunoglobulins to treat the pediatric inflammatory multisystem syndrome-temporally associated with Sars-Cov-2 (PIMS-TS): protocol of the swissped recovery trial. Front. Pediatr. 10, 905046 (2022).
Welzel, T. et al. Methylprednisolone versus intravenous immunoglobulins in children with paediatric inflammatory multisystem syndrome temporally associated with Sars-Cov-2 (PIMS-TS): an open-label, multicentre, randomised trial. Lancet Child Adolesc. Health 7, 238–248 (2023).
Tulling, A. J. et al. Severe pediatric Covid-19 and multisystem inflammatory syndrome in children from wild-type to population immunity: a prospective multicenter cohort study with real-time reporting. Pediatr. Infect. Dis. J. 42, 1077–1085 (2023).
Author information
Authors and Affiliations
Contributions
All listed authors contributed equally to writing and reviewing this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Byrne, D., Gale, C., Canty, N. et al. Neurological and neurodevelopmental effects of Covid and MIS-C on children. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04564-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04564-2