Abstract
Background
We aimed to evaluate the effects of a probiotic preparation containing Lacticaseibacillus rhamnosus MP108 on the improvement of clinical symptoms and gut microbiota in children with Functional Constipation (FC).
Methods
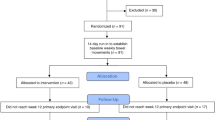
This 4-week randomized, double-blind, placebo-controlled trial assigned 6 to 36-month-old children with FC to supplementation with Lacticaseibacillus rhamnosus MP108 (intervention group, n = 77) or maltodextrin supplementation (control group, n = 77). An electronic questionnaire was used to obtain the defecation status, and 16S rRNA sequencing technology was used to extract the characteristics of gut microbiota. The primary outcomes were treatment success rate (defined as average of ≥3 spontaneous stools movements per week), weekly defecation frequency, and stool hardness. The secondary outcomes included constipation-related symptoms and changes in the gut microbiota. The analysis was performed on an intention-to-treat basis.
Results
After the intervention, the treatment success rate in the intervention group was significantly higher than that in the control group (83.1% vs. 63.6%, P = 0.006). The intervention group demonstrated significantly higher weekly defecation frequency (4.99 ± 2.88 vs. 3.71 ± 2.86, P = 0.002) and Bristol Stool Form Scale (BSFS) scores (3.75 ± 1.04 vs. 2.99 ± 1.17, P = 0.002) compared to the control group. There were significant differences in the gut microbiota, and the intervention group had a higher diversity of gut microbiota Alpha (P = 0.047) and a higher relative abundance of Lacticaseibacillus, Bifidobacteriaceae, Parabacteroides_B_862066; while Erysipelatoclostridium and Eggerthella had lower relative abundance.
Conclusion
Lacticaseibacillus rhamnosus MP108 can effectively improve some constipation symptoms and gut microbiota structure in children with FC.
Impact
-
This is the first study to evaluate the effects of Lacticaseibacillus rhamnosus MP108 on Functional Constipation (FC) in children under 3 years old.
-
The study shows that probiotics containing Lacticaseibacillus rhamnosus MP108 can improve some clinical symptoms and gut microbiota structure in children with FC.
-
The positive results of Lacticaseibacillus rhamnosus MP108 intervention provide new insights for the treatment of FC in children.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon reasonable request to the corresponding author, Q.L. The 16S rRNA sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA1331488.
References
Drossman, D. A. & Hasler, W. L. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology 150, 1257–1261 (2016).
Kilgore, A. & Khlevner, J. Functional constipation: pathophysiology, evaluation, and management. Aliment Pharm. Ther. 60, S20–s29 (2024).
Vriesman, M. H., Koppen, I. J. N., Camilleri, M., Di Lorenzo, C. & Benninga, M. A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 17, 21–39 (2020).
Levy, E. I., Lemmens, R., Vandenplas, Y. & Devreker, T. Functional constipation in children: challenges and solutions. Pediatr. Health Med Ther. 8, 19–27 (2017).
Tran, D. L. & Sintusek, P. Functional constipation in children: what physicians should know. World J. Gastroenterol. 29, 1261–1288 (2023).
Ji, W. J. et al. Epidemiologic survey on the prevalence and distribution of infants’ common gastrointestinal symptoms in 7 cities in china: a population-based study. Zhonghua Liu Xing Bing. Xue Za Zhi 39, 1179–1183 (2018).
Oostenbrink, R., Jongman, H., Landgraf, J. M., Raat, H. & Moll, H. A. Functional abdominal complaints in pre-school children: parental reports of health-related quality of life. Qual. Life Res. 19, 363–369 (2010).
Vriesman, M. H. et al. Quality of life in children with functional constipation: a systematic review and meta-analysis. J. Pediatrics 214, 141–150 (2019).
Benninga, M. A. et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology S0016-5085(16)00182-7 (2016).
Bongers, M. E., Benninga, M. A., Maurice-Stam, H. & Grootenhuis, M. A. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual. Life Outcomes 7, 20 (2009).
Zhou, J., Yuan, X. & Liu, Y. The gut microbiota-constipation connection: insights from a two sample bidirectional Mendelian randomization study. Micro Pathog. 192, 106667 (2024).
Mares, C. R., Săsăran, M. O. & Mărginean, C. O. The relationship between small intestinal bacterial overgrowth and constipation in children—a comprehensive review. Front Cell Infect. Microbiol 14, 1431660 (2024).
Wang, J. et al. Characteristics of the gut microbiome and serum metabolome in patients with functional constipation. Nutrients 15 (2023).
Li, Y. Q. et al. The gut microbiome and metabolites are altered and interrelated in patients with functional constipation. Front. Microbiol. 14, 1320567 (2023).
Villanueva-Millan, M. J. et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am. J. Gastroenterol. 117, 2055–2066 (2022).
Wieërs, G. et al. How probiotics affect the microbiota. Front. Cell Infect. Microbiol. 9, 454 (2019).
Deplancke, B. & Gaskins, H. R. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. Faseb J. 17, 1310–1312 (2003).
Bhattarai, Y. et al. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-ht(3) receptor expression via acetate production. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G80–g87 (2017).
Floch, M. H. Bile salts, intestinal microflora and enterohepatic circulation. Dig. Liver Dis. 34, S54–S57 (2002).
O’Sullivan, J. B., Ryan, K. M., Curtin, N. M., Harkin, A. & Connor, T. J. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int. J. Neuropsychopharmacol. 12, 687–699 (2009).
Doron, S., Snydman, D. R. & Gorbach, S. L. Lactobacillus Gg: bacteriology and clinical applications. Gastroenterol. Clin. North Am. 34, 483–498 (2005). ix.
Succi, M. et al. Bile salt and acid tolerance of lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiol. Lett. 244, 129–137 (2005).
Wang, G. et al. Lactobacillus rhamnosus strains relieve loperamide-induced constipation via different pathways independent of short-chain fatty acids. Front. Cell Infect. Microbiol. 10, 423 (2020).
Zhang, L. L. et al. Lactobacillus Rhamnosus Gg alleviates radiation-induced intestinal injury by modulating intestinal immunity and remodeling gut microbiota. Microbiol Res. 286, 127821 (2024).
Sepp, E., Mikelsaar, M. & Salminen, S. Effect of administration of Lactobacillus casei strain gg on the gastrointestinal microbiota of newborns. Microb. Ecol. Health Dis. 6, 309–314 (1993).
Duan, C. et al. Lactobacillus Rhamnosus attenuates intestinal inflammation induced by Fusobacterium nucleatum infection by restoring the autophagic flux. Int. J. Mol. Med. 47, 125–136 (2021).
Lo Vecchio, A., Nunziata, F., Bruzzese, D., Conelli, M. L. & Guarino, A. Rotavirus immunisation status affects the efficacy of Lacticaseibacillus Rhamnosus Gg for the treatment of children with acute diarrhoea: a meta-analysis. Benef. Microbes 13, 283–294 (2022).
Luoto, R., Pärtty, A., Vogt, J. K., Rautava, S. & Isolauri, E. Reversible aberrancies in gut microbiome of moderate and late preterm infants: results from a randomized, controlled trial. Gut Microbes 15, 2283913 (2023).
Zhang, B., Lynch, B., Zhao, J., Guo, Y. & Mak, A. Lactobacillus Rhamnosus Mp108: toxicological evaluation. J. Food Sci. 86, 228–241 (2021).
Yan, H. D. et al. Study on the synergistic regulation of immune function in rats by oligosaccharides and Lactobacillus Rhamnosus Mp108. Food Ferment. Industries, 1-11.
Zhao, Y. M., Hou, Y. M. & Zhao, Y. Q. Study of Lacticaseibacillus Rhamnosus Mp108 on alleviating bacterial diarrhea. Sci. Technol. Food Ind. 43, 20–27 (2022).
Zhang, C. et al. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin. Nutr. 39, 2960–2969 (2020).
Wegh, C. A. M., Benninga, M. A. & Tabbers, M. M. Effectiveness of probiotics in children with functional abdominal pain disorders and functional constipation: a systematic review. J. Clin. Gastroenterol. 52, S10-s26 (2018).
Wojtyniak, K., Horvath, A., Dziechciarz, P. & Szajewska, H. Lactobacillus Casei Rhamnosus Lcr35 in the management of functional constipation in children: a randomized trial. J. Pediatr. 184, 101–105.e101 (2017).
Quigley, E. M., Vandeplassche, L., Kerstens, R. & Ausma, J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation-a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharm. Ther. 29, 315–328 (2009).
Chumpitazi, B. P. et al. Bristol stool form scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol. Motil. 28, 443–448 (2016).
Malhotra, A. et al. Use of Bristol Stool Form Scale to predict the adequacy of bowel preparation—a prospective study. Colorectal Dis. 18, 200–204 (2016).
Blake, M. R., Raker, J. M. & Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharm. Ther. 44, 693–703 (2016).
Gao, Y., Zhang, G., Jiang, S. & Liu, Y. X. Wekemo bioincloud: a user-friendly platform for meta-omics data analyses. Imeta 3, e175 (2024).
Wallace, C. et al. Probiotics for treatment of chronic constipation in children. Cochrane Database Syst. Rev. 3, Cd014257 (2022).
Callahan, B. J. et al. Dada2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
DeSantis, T. Z. et al. Greengenes, a Chimera-checked 16s rRNA gene database and workbench compatible with Arb. Appl Environ. Microbiol 72, 5069–5072 (2006).
Marungruang, N., Tovar, J., Björck, I. & Hållenius, F. F. Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects. Eur. J. Nutr. 57, 2927–2936 (2018).
Gu, Y. et al. Lactobacillus Rhamnosus Gg supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 13, 12144–12155 (2022).
Li, H. et al. Lactobacillus Rhamnosus Mp108 alleviates ulcerative colitis in mice by enhancing the intestinal barrier, inhibiting inflammation, and modulating gut microbiota. Food Science Human Wellness (2024).
Bu, L. N., Chang, M. H., Ni, Y. H., Chen, H. L. & Cheng, C. C. Lactobacillus Casei Rhamnosus Lcr35 in children with chronic constipation. Pediatr. Int. 49, 485–490 (2007).
Basturk, A., Isik, İ, Atalay, A. & Yılmaz, A. Investigation of the efficacy of Lactobacillus Rhamnosus Gg in infants with cow’s milk protein allergy: a randomised double-blind placebo-controlled trial. Probiotics Antimicrob. Proteins 12, 138–143 (2020).
Banaszkiewicz, A. & Szajewska, H. Ineffectiveness of Lactobacillus Gg as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J. Pediatr. 146, 364–369 (2005).
Coccorullo, P. et al. Lactobacillus Reuteri (Dsm 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo-controlled study. J. Pediatr. 157, 598–602 (2010).
Jung, C. et al. Effect of L. Reuteri on bowel movements in children aged 6 months to 4 years: a double-blind randomized controlled trial. Front Pediatr. 10, 997104 (2022).
Sadeghzadeh, M., Rabieefar, A., Khoshnevisasl, P., Mousavinasab, N. & Eftekhari, K. The effect of probiotics on childhood constipation: a randomized controlled double blind clinical trial. Int. J. Pediatr. 2014, 937212 (2014).
Huang, L., Zhu, Q., Qu, X. & Qin, H. Microbial treatment in chronic constipation. Sci. China Life Sci. 61, 744–752 (2018).
Zeng, X. L., Yang, X. D., Yang, T., Huang, X. L. & Liu, S. Etiology and clinical classification of constipation. Zhonghua Wei Chang Wai Ke Za Zhi 25, 1120–1125 (2022).
[Chinese Expert Consensus on the Diagnosis and Treatment of Outlet Obstructive Constipation (2022 Edition)]. Zhonghua Wei Chang Wai Ke Za Zhi 25, 1045-1057 (2022).
Wang, Y. B. et al. The evaluation of Gi-Pill gastrointestinal electronic capsule for colonic transit test in patients with slow transit constipation. Int J. Colorectal Dis. 35, 29–34 (2020).
Xie, S. K. et al. Changes of intestinal flora and effects of corresponding intervention in colon slow transit constipation rats. Guangdong Med. J. 37, 325–327 (2016).
Kumar, A. et al. Probiotics as modulators of gut-brain axis for cognitive development. Front Pharm. 15, 1348297 (2024).
Šola, K. F. et al. The effect of multistrain probiotics on functional constipation in the elderly: a randomized controlled trial. Eur. J. Clin. Nutr. 76, 1675–1681 (2022).
Cheng, J. et al. Eight-week supplementation with bifidobacterium lactis hn019 and functional constipation: a randomized clinical trial. JAMA Netw. Open 7, e2436888 (2024).
Wallace, C., Gordon, M., Sinopoulou, V. & Akobeng, A. K. Probiotics for management of functional abdominal pain disorders in children. Cochrane Database Syst. Rev. 2, Cd012849 (2023).
Ki Cha, B. et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 46, 220–227 (2012).
Kwon, H. et al. Effect of Lacticaseibacillus Rhamnosus Idcc 3201 on irritable bowel syndrome with constipation: a randomized, double-blind, and placebo-controlled trial. Sci. Rep. 14, 22384 (2024).
Chantanawilas, P., Pahumunto, N., Thananimit, S. & Teanpaisan, R. Anticandidal activity of various probiotic Lactobacillus strains and their efficacy enhanced by prebiotic supplementation. Curr. Microbiol 81, 271 (2024).
Peng, Y. et al. Lactobacillus Reuteri in digestive system diseases: focus on clinical trials and mechanisms. Front Cell Infect. Microbiol. 13, 1254198 (2023).
Mathipa-Mdakane, M. G. & Thantsha, M. S. Lacticaseibacillus Rhamnosus: a suitable candidate for the construction of novel bioengineered probiotic strains for targeted pathogen control. Foods 11 (2022).
Qin, W. et al. Integrative Atac-Seq and RNA-Seq analyses of Ipec-J2 Cells reveals porcine transcription and chromatin accessibility changes associated with Escherichia Coli F18ac inhibited by Lactobacillus Reuteri. Front Microbiol 14, 1101111 (2023).
Chantanawilas, P., Pahumunto, N. & Teanpaisan, R. Aggregation and adhesion ability of various probiotic strains and Candida species: an in vitro study. J. Dent. Sci. 19, 2163–2171 (2024).
Park, M. A. et al. Anti-inflammatory potential via the MAPK signaling pathway of Lactobacillus spp. isolated from canine feces. PLoS One 19, e0299792 (2024).
Jeong, M. et al. Heat-killed Lactobacillus Brevis enhances phagocytic activity and generates immune-stimulatory effects through activating the Tak1 pathway. J. Microbiol. Biotechnol. 30, 1395–1403 (2020).
Asare, P. T. et al. Reuterin demonstrates potent antimicrobial activity against a broad panel of human and poultry meat Campylobacter Spp. isolates. Microorganisms 8 (2020).
Thierry, A. et al. New insights into physiology and metabolism of Propionibacterium Freudenreichii. Int. J. Food Microbiol. 149, 19–27 (2011).
Arvans, D. L. et al. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G696–G704 (2005).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Ezeji, J. C. et al. Parabacteroides distasonis: intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 13, 1922241 (2021).
Cui, Y. et al. Roles of intestinal parabacteroides in human health and diseases. FEMS Microbiol. Lett. 369, fnac072 (2022).
Cai, S. et al. Gut bacteria Erysipelatoclostridium and its related metabolite ptilosteroid a could predict radiation-induced intestinal injury. Front. Public Health 10, 862598 (2022).
Zhu, X. et al. A high-fat diet increases the characteristics of gut microbial composition and the intestinal damage associated with non-alcoholic fatty liver disease. Int. J. Mol. Sci. 24, 16733 (2023).
Zorkina, Y. A. et al. [Effects of diet on the gut microbiome in patients with depression]. Zh. Nevrol. Psikhiatr Im. S S Korsakova 122, 59–64 (2022).
Zhang, W., Xu, X., Cai, L. & Cai, X. Dysbiosis of the gut microbiome in elderly patients with hepatocellular carcinoma. Sci. Rep. 13, 7797 (2023).
Alexander, M. et al. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe 30, 17–30.e19 (2022).
Milani, C. et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036-17 (2017).
Acknowledgements
We would like to thank all the researchers involved in the investigation for their help, as well as the leaders and colleagues of the Maternal and Child Health Hospital and the Children’s Hospital for their strong support, as well as the participation of the study subjects. This research was funded by the Natural Science Foundation of Hunan Province (grant number: 2025JJ50562).
Author information
Authors and Affiliations
Contributions
Significant contributions to concept and design, data analysis and interpretation: X.P., Y.P., M.W.; Important contributions to data acquisition: X.P., Y.P., H.Z., M.W., Q.X., X.S., W.L., Y.L., W.X.; Major contributions to probiotic research and development: Y.Z., W.L.; Drafting articles or critically revising important knowledge content: Q.L., H.Z., X.P., Y.P.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Consent statement
After being fully informed about the study, primary care givers consent to their children’s participation. Before the study began, each participant’s parents provided written informed consent. The study was approved by the Ethics Committee of Xiangya School of Public Health of Central South University, the Ethics Committee of Changsha Maternal and Child Health Hospital and the Ethics Committee of Hunan Children’s Hospital.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, C., Pan, Y., Wu, M. et al. Effects of Lacticaseibacillus rhamnosus MP108 on functional constipation symptoms and gut microbiota in children. Pediatr Res (2026). https://doi.org/10.1038/s41390-025-04567-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04567-z