Abstract
Background
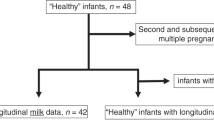
Immunoglobulin A (IgA) binding gut bacteria may modulate necrotising enterocolitis (NEC). In infants <32 weeks gestation exclusively receiving their mother’s own milk (MOM) who either did or did not develop NEC, we explored IgA concentration in MOM and infant stool.
Methods
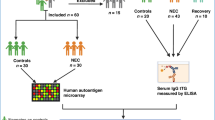
We quantified IgA and secretory IgA (sIgA) concentration by enzyme linked immunosorbent assay (ELISA). 41 NEC (median onset day 22) and 44 matched control infants, median gestation 25 weeks, contributed 202 MOM and 92 stool samples.
Results
IgA and sIgA in MOM were significantly lower for the first three weeks in infants developing NEC compared to control infants (median IgA 499 μg/ml vs 1261 µg/ml, sIgA 481 µg/ml versus 1023 µg/ml, adjusted p < 0.001 between 0–6 and 7–13 days of life, adjusted p = 0.029 days 14–20). Stool IgA concentration before disease was also significantly lower in NEC infants compared with control infants (262 µg/ml versus 491 µg/ml, p = 0.045), but there was no statistical difference in sIgA.
Conclusion
IgA and sIgA are lower in early MOM (before week 3 of life) received by infants who go on to develop NEC, reflected in lower stool IgA. Potential mechanisms require further elucidation. Targeted use of high IgA donor milk to preterm infants may be beneficial.
Impact
-
This is the first published work that evaluates both milk and stool IgA and sIgA in control preterm infants and those developing NEC and has the benefit of samples taken before disease onset for some infants.
-
In this study, IgA and sIgA are lower in early MOM (before week 3 of life) received by infants who go on to develop NEC, reflected in lower stool IgA.
-
Targeted use of high IgA donor milk to preterm infants may be beneficial.
-
Clinical practice in the interim should focus on maximising fresh MOM delivery to preterm babies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Dermyshi, E., Granger, C., Chmelova, K., Embleton, N. & Berrington, J. Age of onset of necrotising enterocolitis (NEC) and focal intestinal perforation (FIP) in very preterm and low birthweight infants: A systematic review. BMJ Open 24, e070638 (2023).
Battersby, C., Longford, N., Mandalia, S., Costeloe, K. & Modi, N. UK Neonatal Collaborative Necrotising Enterocolitis (UKNC-NEC) study group. Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012–13: A whole-population surveillance study. Lancet Gastroenterol. Hepatol. 2, 43–51 (2017).
Masi, A. C. et al. Human milk microbiota, oligosaccharide profiles, and infant gut microbiome in preterm infants diagnosed with necrotizing enterocolitis. Cell Rep. Med 17, 101708 (2024).
Granger, C. L. et al. Maternal breastmilk, infant gut microbiome and the impact on preterm infant health. Acta Paediatr. 110, 450–457 (2021).
Masi, A. C. et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut 70, 2273–2282 (2021).
Autran, C. A. et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 67, 1064–1070 (2018).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Granger, C. L. et al. Secretory immunoglobulin A in preterm infants: determination of normal values in breast milk and stool. Pediatr. Res. 92, 979–986 (2022).
Mantis, N. J., Rol, N. & Corthesy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611 (2011).
Demers-Mathieu, V. et al. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients 10, 631 (2018).
Palmeira, P. & Carneiro-Sampaio, M. Immunology of breast milk. Rev. Assoc. Med. Bras. 62, 584–593 (2016).
Bunker, J. J. et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 15, 541–553 (2015).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 28, 1000–1010 (2014).
Kubinak, J. L. et al. MyD88 signalling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe 17, 153–163 (2015).
Berrington, J. & Embleton, N. D. Discriminating necrotising enterocolitis and focal intestinal perforation. Arch. Dis. Child. Fetal Neonatal Ed. 107, 336–339 (2022).
Pietrasanta, C. et al. Prenatal antibiotics reduce breast milk IgA and induce dysbiosis in mouse offspring, increasing neonatal susceptibility to bacterial sepsis. Cell Host Microbe 11, 2178–2194 (2024).
Eibl, M. M., Wolf, H. M. & Fürnkranz, H. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA-IgG feeding. N. Engl. J. Med. 319, 1–7 (1988).
Foster, J. P., Seth, R. & Cole, M. J. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates. Cochrane Database Syst. Rev. 4, CD001816 (2016).
Acknowledgement
N.D.E. and J.E.B. declare institutional research funding from Prolacta Biosciences and Danone Early Life Nutrition, and honoraria from Danone Early Life Nutrition, and Nestle Nutrition Institute. C.J.S. declares receiving lecture honoraria from Nestle Nutrition Institute but has no share options or other conflicts. C.L.G. has obtained a grant from the Human Milk Foundation (HMF), which partly funded this research. The other authors declare that they have no competing interests. C.A.L. is supported by the NIHR Newcastle Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
C.L.G., C.A.L., N.D.E., J.M.P., C.J.S. and J.E.B. were responsible for the study concept, design and methodology. Wet lab work was performed by C.L.G. supported by J.M.P. Data analysis and interpretation was led by C.L.G. and A.C.M. and supported by all authors. C.L.G., A.C.M., and J.E.B. drafted the manuscript with subsequent critical revisions and edits from all authors. All authors approved the final manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Participants were recruited to an ongoing REC approved sample salvage study (SERVIS Supporting Research in Vulnerable Infants REC 10/H0908/39). No additional consent was required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Granger, C.L., Masi, A.C., Lamb, C.A. et al. Immunoglobulin A concentration is lower in mothers’ own milk and infant stool in infants who develop necrotising enterocolitis. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04570-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04570-4