Abstract
Background
Neonatal encephalopathy (NE) is a leading cause of neonatal mortality and disability. The study aims to analyze the condition’s global burden and temporal trends.
Methods
Data were extracted from the Global Burden of Disease (GBD) 2021 database, mainly on the cases and rate of prevalence, disability-adjusted life years (DALYs), and deaths of NE from 1991 to 2021. We analyzed prevalence, DALYs, and mortality at different levels. Trends were quantified using percentage change and estimated annual percentage change (EAPC).
Results
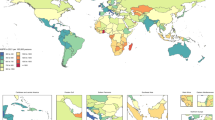
Globally, the prevalence of NE has increased from 1991 to 2021, while DALYs and deaths rates decreased. Low Socio-Demographic Index (SDI) regions had the highest prevalence cases in 2021 (15432, 95%UI: 12884–18173). The largest increase of prevalence occurred in South Asia over 31 years, with an EAPC of 2.11 (95%CI: 1.99–2.22). Ethiopia exhibited the largest increase in prevalent cases and rates, with a percentage of 248.51% and an EAPC of 3.56 (95%CI: 3.33–10.92). The burden of NE was consistently higher in males than in females.
Conclusion
Despite global improvements in DALYs and mortality, the increasing prevalence of NE in certain regions highlights the need for targeted public health strategies, particularly in low SDI regions.
Impact Statement
-
We estimated the temporal trends of prevalence, disability-adjusted life years (DALYs), and death following neonatal encephalopathy (NE).
-
The results demonstrated that NE increased, while DALYs and death decreased globally from 1991 to 2021.
-
The prevalence, DALYs, and death rates for NE exhibited a negative correlation with the Socio-Demographic Index (SDI), suggesting a progressive reduction in disease burden as regional socioeconomic conditions improve.
-
Targeted public health strategies are required to implemented in different SDI regions, improving the burden of NE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data and relevant analyses in this study were available in the online sources of the GBD 2021 database. (https://vizhub.healthdata.org/gbd-results/).
References
Sandoval Karamian, A. G. et al. Neonatal encephalopathy: Etiologies other than hypoxic-ischemic encephalopathy. Semin Fetal Neonatal Med 26, 101272 (2021).
Ding, C. et al. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990-2019. Front Public Health 10, 952161 (2022).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 74, 50–72 (2013).
Tuura, R. O. et al. Elevated cerebral perfusion in neonatal encephalopathy is associated with neurodevelopmental impairments. Pediatr Res https://doi.org/10.1038/s41390-024-03553-1 (2024).
GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2133–2161 (2024).
Huang, D. et al. Global temporal trends and projections of acute hepatitis E incidence among women of childbearing age: Age-period-cohort analysis 2021. J Infect 89, 106250 (2024).
Li, X. et al. Sociodemographic index-age differences in the global prevalence of cardiovascular diseases, 1990-2019: a population-based study. Arch Public Health 83, 2 (2025).
Gebeyehu, D. T., East, L., Wark, S. & Islam, M. S. Disability-adjusted life years (DALYs) based COVID-19 health impact assessment: a systematic review. BMC Public Health 23, 334 (2023).
Cen, J. et al. Global, regional, and national burden and trends of migraine among women of childbearing age from 1990 to 2021: insights from the Global Burden of Disease Study 2021. J Headache Pain 25, 96 (2024).
Wang, R., Li, Z., Liu, S. & Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
Ambrose, A. et al. Neonatal Encephalopathy: Novel Phenotypes and Genotypes Identified by Genome Sequencing. Neurol Genet 11, e200232 (2025).
Tang, Z., Mahmoodi, S., Darekar, A. & Vollmer, B. Automatic veins analysis of susceptibility weighted image in hypoxic-ischaemic encephalopathy. Magn Reson Imaging 98, 83–96 (2023).
Zheng, Q. et al. Cerebral Pulsed Arterial Spin Labeling Perfusion Weighted Imaging Predicts Language and Motor Outcomes in Neonatal Hypoxic-Ischemic Encephalopathy. Front Pediatr 8, 576489 (2020).
Li, H. X. et al. Resting-state network complexity and magnitude changes in neonates with severe hypoxic ischemic encephalopathy. Neural Regen Res 14, 642–648 (2019).
Kansagra, A. P. et al. Microstructural maturation of white matter tracts in encephalopathic neonates. Clin Imaging 40, 1009–1013 (2016).
Hermans, T. et al. Partial wavelet coherence as a robust method for assessment of neurovascular coupling in neonates with hypoxic ischemic encephalopathy. Sci Rep 13, 457 (2023).
Tsuda, K. et al. Three-year outcome following neonatal encephalopathy in a high-survival cohort. Sci Rep 12, 7945 (2022).
Laptook, A. R. et al. Effect of Therapeutic Hypothermia Initiated After 6 h of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA 318, 1550–1560 (2017).
Simbruner, G., Mittal, R. A., Rohlmann, F. & Muche, R. neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126, e771–e778 (2010).
Garnaud, H., Cressens, S., Arbaoui, H. & Ayachi, A. Servo-controlled therapeutic hypothermia during neonatal transport: a before-and-after quality improvement project. Eur J Pediatr 183, 4259–4264 (2024).
Goel, N., Mohinuddin, S. M., Ratnavel, N., Kempley, S. & Sinha, A. Comparison of Passive and Servo-Controlled Active Cooling for Infants with Hypoxic-Ischemic Encephalopathy during Neonatal Transfers. Am J Perinatol 34, 19–25 (2017).
Thayyil, S. et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health 9, e1273–e1285 (2021).
Naburi, H. E. et al. A survey on the diagnosis and management of neonatal hypoxic ischaemic encephalopathy in sub-saharan Africa. Sci Rep 14, 22046 (2024).
Krishnan, V., Kumar, V., Shankaran, S. & Thayyil, S. Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial. Indian J Pediatr https://doi.org/10.1007/s12098-021-03861-y (2021).
Oliveira, R. F., Wagner, L. H., Silva, A. S. D., Rodrigues, M. C. C. & Lacerda, G. C. B. Neurological outcomes in neonates treated with therapeutic hypothermia: challenges in a developing country. Arq Neuropsiquiatr 82, 1–8 (2024).
Wu, Y. W. et al. Nighttime delivery and risk of neonatal encephalopathy. Am J Obstet Gynecol 204, e1–e6 (2011).
Kelly, L. A., Branagan, A., Semova, G. & Molloy, E. J. Sex differences in neonatal brain injury and inflammation. Front Immunol 14, 1243364 (2023).
Lang, J. T. & McCullough, L. D. Pathways to ischemic neuronal cell death: are sex differences relevant?. J Transl Med 6, 33 (2008).
Chanana, V. et al. Sex Differences in Mouse Hippocampal Astrocytes after In-Vitro Ischemia. J Vis Exp 116, 53695 (2016).
Dietz, R. M. et al. Therapeutic hypothermia protects against ischemia-induced impairment of synaptic plasticity following juvenile cardiac arrest in sex-dependent manner. Neuroscience 325, 132–141 (2016).
Uluc, K. et al. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol Disord Drug Targets 12, 360–370 (2013).
Montaldo, P. et al. Whole-Blood Gene Expression Profile After Hypoxic-Ischemic Encephalopathy. JAMA Netw Open 7, e2354433 (2024).
Vidavalur, R. & Bhutani, V. K. Neonatal encephalopathy in India: spatiotemporal variations in declining mortality. Pediatr Res. https://doi.org/10.1038/s41390-025-04009-w
Nushrat, K. et al. A scoping review and modelling of predictors of an abnormal Thompson score in term neonates in low-resource settings. Sci Rep 15, 12217 (2025).
Shrestha, S., Dhungana, S. P., Shrestha, S. & Shrestha, G. S. Correlation of Thompson Score in Predicting Early Outcome of Newborn with Birth Asphyxia. J Nepal Health Res Counc 18, 406–410 (2020).
Mendler, M. R. et al. Predictive Value of Thompson-Score for Long-Term Neurological and Cognitive Outcome in Term Newborns with Perinatal Asphyxia and Hypoxic-Ischemic Encephalopathy Undergoing Controlled Hypothermia Treatment. Neonatology 114, 341–347 (2018).
Duran, R., Aladağ, N., Vatansever, U., Süt, N. & Acunaş, B. The impact of Neonatal Resuscitation Program courses on mortality and morbidity of newborn infants with perinatal asphyxia. Brain Dev 30, 43–46 (2008).
Bonifacio, S. L. & Hutson, S. The Term Newborn: Evaluation for Hypoxic-Ischemic Encephalopathy. Clin Perinatol. 48, 681–695 (2021).
Mathieson, S. R. et al. EEG background activity, seizure burden and early childhood outcomes in neonatal encephalopathy in Uganda: a prospective feasibility cohort study. EClinicalMedicine 78, 102937 (2024).
Vidavalur, R., More, K. & Bhutani, V. K. Assessment of Global Burden due to neonatal encephalopathy: An economic evaluation. Semin Fetal Neonatal Med 29, 101560 (2024).
Hilker, S. et al. A Proof-of-Concept Model for Implementing a “Smart-NICU” to Improve Infant Mortality. J Intensive Care Med https://doi.org/10.1177/08850666241247532 (2024).
Acknowledgements
The authors appreciate the works of the GBD Study 2021 collaborators. No financial assistance was received.
Author information
Authors and Affiliations
Contributions
Z.L. contributed to the conception and design of the study, statistical analysis, and drafting the manuscript. J.Y.Y. and Z.Z.Y. contributed to the methodology and statistical analysis. W.L.S. contributed to the conception and supervision, and revised and commented on the draft. This study was completed with the assistance and approval of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Jiang, Y., Zhao, Z. et al. Global, regional, and national burden and trends of neonatal encephalopathy. Pediatr Res (2026). https://doi.org/10.1038/s41390-025-04582-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04582-0
This article is cited by
-
Long-term disability after neonatal encephalopathy in low-resource settings
Pediatric Research (2026)