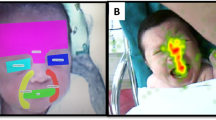
Abstract
Background
Neonates in intensive care undergo an average of 13 painful procedures daily, with untreated pain linked to structural brain alterations and long-term cognitive and behavioral impairments. Current pain assessment relies on subjective evaluation scales that vary according to infant characteristics, procedure type, and evaluator background, highlighting the need for more objective assessment methods.
Methods
We leverage a Vision-Language Model (VLM) for neonatal Automatic Pain Assessment (APA) and implemented novel prompt categories based on two approaches: (1) encouraging the model to retrieve clinical knowledge from its pretraining, and (2) providing information about clinically relevant facial features.
Results
When leveraging latent clinical knowledge, the model achieved a balance of precision (82.3%) and recall (73.2%). When assessing clinically relevant facial features, it reached perfect precision (100%) with lower recall (40.1%).
Conclusion
This first application of VLMs for neonatal APA demonstrates superior performance compared to previous deep learning approaches. The model effectively retrieves latent clinical knowledge and performs best when provided with clinical context. When instructed with specific criteria and facial features, it achieved high precision with significantly reduced withdrawal rates compared to baseline prompts, highlighting the feasibility of this novel approach as a real-world evaluation of state-of-the-art technology through all its steps of development.
Impact
-
This study pioneers the application of Vision-Language Models (VLMs) for Automatic Pain Assessment in neonates, offering a novel alternative to traditional deep learning approaches.
-
We demonstrate that carefully designed prompts can leverage a model’s latent clinical knowledge or guide it to assess specific facial features, without requiring fine-tuning.
-
Our approach achieves perfect precision (100%) when assessing clinically relevant facial features, surpassing previous deep learning methods.
-
This work opens new research directions for neonatal pain assessment using instruction-based AI systems that can incorporate clinical expertise through natural language prompts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The complete database is available on request with the corresponding authors of them.
References
Wang, Y. et al. Factors influencing the occurrence of neonatal procedural pain. J. Spec. Pediatr. Nurs. 25, e12281 (2020).
Boggini, T. et al. Cumulative procedural pain and brain development in very preterm infants: A systematic review of clinical and preclinical studies. Neurosci. Biobehav Rev. 123, 320–336 (2021).
Grunau, R. E., Oberlander, T., Holsti, L. & Whitfield, M. F. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 76, 277–286 (1998).
Giordano, V. et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatr. 173, 1186–1197 (2019).
Balda, R. C. X., Almeida, M. F. B., Peres, C. A. & Guinsburg, R. Factors that interfere in the recognition of the neonatal facial expression of pain by adults. Rev. Paul. Pediatr. 27, 160–167 (2009).
Carpentier, E., Moreau, F., Soriot-Thomas, S. & Tourneux, P. Training program for pain assessment in the newborn. Arch. de. Pédiatr. 25, 35–38 (2018).
Cascella, M. et al. Artificial intelligence for automatic pain assessment: research methods and perspectives. Pain. Res. Manag. 2023, 6018736 (2023).
Zhang, M. et al. Using artificial intelligence to improve pain assessment and pain management: a scoping review. J. Am. Med. Inform. Assoc. 30, 570–587 (2023).
Heiderich, T. M. et al. Face-based automatic pain assessment: challenges and perspectives in neonatal intensive care units. J. Pediatr. 99, 546–560 (2023).
Brahnam, S., Chuang, C. F., Shih, F. Y. & Slack, M. R. Machine recognition and representation of neonatal facial displays of acute pain. Artif. Intell. Med. 36, 211–222 (2006).
Zamzmi, G., Goldgof, D., Kasturi, R., Sun, Y. Neonatal pain expression recognition using transfer learning. arXiv preprint arXiv: 1807.01631 (2018)
Carlini, L. P. et al A convolutional neural network-based mobile application to bedside neonatal pain assessment, in: 2021 34th SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), IEEE Computer Society. pp. 394–401 (2021).
Salekin, M. S. et al. Multimodal spatio-temporal deep learning approach for neonatal postoperative pain assessment. Comput. Biol. Med. 129, 104150 (2021).
Coutrin, G. A. et al. Convolutional neural networks for newborn pain assessment using face images: A quantitative and qualitative comparison, in: Proceedings of the 3rd International Conference on Medical Imaging and Computer-Aided Diagnosis, MICAD 2022, Springer LNEE (2022).
Carlini, L. P. et al. Human vs machine towards neonatal pain assessment: A comprehensive analysis of the facial features extracted by health professionals, parents, and convolutional neural networks. Artif. Intell. Med. 147, 102724 (2024).
Schmidt, D. C., Spencer-Smith, J., Fu, Q., White, J. Towards a catalog of prompt patterns to enhance the discipline of prompt engineering (2023).
Wei, J. et al. Chain-of-thought prompting elicits reasoning in large language models. Adv. neural Inf. Process. Syst. 35, 24824–24837 (2022).
Hartsock, I. & Rasool, G. Vision-language models for medical report generation and visual question answering: a review. Front. Artif. Intell. 7, 1430984 (2024).
Qin, Z., Yi, H., Lao, Q., Li, K. Medical image understanding with pretrained vision language models: A comprehensive study. arXiv preprint arXiv:2209.15517 (2022)
Liévin, V., Hother, C. E., Motzfeldt, A. G. & Winther, O. Can large language models reason about medical questions? Patterns 5, 100943 (2024).
Perlis, R. H., Goldberg, J. F., Ostacher, M. J., Schneck, C. D. Clinical decision support for bipolar depression using large language models. Neuropsychopharmacology, 1–5 (2024).
Grunau, R. V. & Craig, K. D. Pain expression in neonates: facial action and cry. Pain 28, 395–410 (1987).
Barros, M. C. D. M. et al. Identification of pain in neonates: the adults’ visual perception of neonatal facial features. J. Perinatol. 41, 2304–2308 (2021).
Kong, A. et al Better zero-shot reasoning with role-play prompting, in Proceedings of the 2024 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies (Volume 1: Long Papers), pp. 4099–4113 (2024).
Lawrence, J. et al. The development of a tool to assess neonatal pain. Neonatal Netw.: NN 12, 59–66 (1993).
Krechel, S. W. & Bildner, J. Cries: a new neonatal postoperative pain measurement score. initial testing of validity and reliability. Pediatr. Anesth. 5, 53–61 (1995).
Stevens, B., Johnston, C., Petryshen, P. & Taddio, A. Premature infant pain profile: development and initial validation. Clin. J. pain. 12, 13–22 (1996).
Brahnam, S., Chuang, C. F., Shih, F. Y., Slack, M. R. Svm classification of neonatal facial images of pain. International Workshop on Fuzzy Logic and Applications, 121–128 (2005).
Heiderich, T. M., Leslie, A. T. F. S. & Guinsburg, R. Neonatal procedural pain can be assessed by computer software that has good sensitivity and specificity to detect facial movements. Acta Paediatr. 104, e63–e69 (2015).
Deng, J., Guo, J., Zhou, Y., Yu, J., Kotsia, I., Zafeiriou, S. Retinaface: Single-stage dense face localisation in the wild. arXiv:1905.00641 (2019).
Team, G., Anil, R., Borgeaud, S., Alayrac J.-B., e.a. Gemini: A family of highly capable multimodal models. URL: https://arxiv.org/abs/2312.11805, arXiv:2312.11805 (2024).
Grunau, R. V., Johnston, C. C. & Craig, K. D. Neonatal facial and cry responses to invasive and non-invasive procedures. Pain 42, 295–305 (1990).
Hurst, A. et al. GPT-4o system card. arXiv preprint arXiv:2410.21276 (2024).
Anthropic. The Claude 3 model family: Opus, Sonnet, Haiku. URL: https://www-cdn.anthropic.com/de8ba9b01c9ab7cbabf5c33b80b7bbc618857627/Model_Card_Claude_3.pdf (2024).
Funding
The authors would like to thank the financial support provided by the University College FEI, and the Brazilian funding agencies FAPESP (2018/13076-9), CNPq (401059/2019-7), and CAPES.
Author information
Authors and Affiliations
Contributions
L.P.C.: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing – original draft. G.deA.SáC.: Data curation; Formal analysis; Investigation; Methodology; Validation, Visualization; Writing – review & editing. L.A.F.: Data curation; Formal analysis; Investigation; Methodology; Validation, Visualization; Writing – review & editing. T.M.H.: Investigation. R.deC.X.B.: Supervision. M.C.deM.B.: Conceptualisation; Formal Analysis; Project administration; Supervision. R.G.: Conceptualisation; Formal Analysis; Project administration; Supervision; Writing - review & editing. C.E.T.: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Corresponding author
Ethics declarations
Consent for Publication
This manuscript has been submitted only to Pediatric Research and it will not be submitted elsewhere while under consideration. All authors listed on the manuscript approved the submission of this version of the manuscript and take full responsibility for the manuscript.
Competing interests
The authors declare no competing interests.
Ethics approval
Our research does not need an ethics statement, because all the data used are benchmark databases already approved by the appropriate institutional committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira Carlini, L., Antunes Ferreira, L., de Almeida Sá Coutrin, G. et al. Is this neonate feeling pain? Leveraging clinical knowledge towards high-precision Large Language Model-based neonatal pain assessment. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04669-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04669-8