Abstract
Background
Umbilical cord blood (UCB) is increasingly studied for regenerative therapies, yet the impact of different collection techniques on cell yield and quality remains unclear. This study compared standard needle-and-bag UCB collection with manual cord milking, performed both in utero (placenta attached) and ex utero (placenta delivered), using samples from healthy term infants ( > 37 weeks gestation).
Method
Forty-two samples (n = 10 standard in utero, n = 10 standard ex utero, n = 10 milking in utero, n = 12 milking ex utero) were analyzed for blood volume, mononuclear cell count, and cellular composition via flow cytometry. Key cell populations included hematopoietic stem cells (CD34 + CD45 + ), endothelial progenitor cells (CD45 + CD34 + CD31 − CD133 + ), and mature endothelial cells (CD34 − CD45 − CD31 + ). Plasma cytokines, including inflammatory and angiogenic markers, were also assessed.
Results
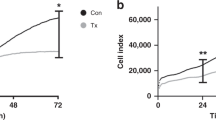
No significant differences were found in total blood volume or mononuclear cell counts across groups. However, endothelial progenitor cell viability was significantly reduced in cord milking ex utero compared to standard in utero collection (p < 0.0001). Cytokine analysis showed elevated IL-1RA and reduced VEGF-A in cord milking ex utero samples (p < 0.0001 and p = 0.0004, respectively).
Conclusion
These findings suggest that in utero cord milking may be a viable alternative to standard UCB collection, preserving cell viability and cytokine integrity.
Impact
-
The standard method for collecting umbilical cord blood (UCB) has limitations, especially in cases like premature birth, where low volumes yield insufficient mononuclear and hematopoietic stem cells for therapeutic use.
-
This study evaluated an alternative technique—umbilical cord milking—against the standard approach.
-
As the first study to assess its efficacy for UCB collection, the findings offer insights into a viable alternative method.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data is available on reasonable request from authors.
References
Gupta, A. O. & Wagner, J. E. Umbilical cord blood transplants: current status and evolving therapies. Front. Pediatr. 8, 570282 (2020).
McDonald, C. A., Fahey, M. C., Jenkin, G. & Miller, S. L. Umbilical cord blood cells for treatment of cerebral palsy; timing and treatment options. Pediatr. Res 83, 333–344 (2018).
Cohen, S. et al. Single Um171 expanded cord blood transplant is feasible, safe, and permits transplantation of better Hla Matched cords with very low transplant related mortality. Blood 130, 658–658 (2017).
Geneugelijk, K. & Spierings, E. Immunogenetic factors in the selection of cord blood units for transplantation: current search strategies and future perspectives. Cytotherapy 17, 702–710 (2015).
Wang, J. & Metheny, L. Umbilical cord blood derived cellular therapy: advances in clinical development. Front. Oncol. 13, 1167266 (2023).
Nguyen, T. et al. Umbilical cord blood-derived cell therapy for perinatal brain injury: a systematic review & meta-analysis of preclinical studies. Int. J. Mol. Sci. 24, 4351 (2023).
Li, J. et al. Term Vs. Preterm cord blood cells for the prevention of preterm brain injury. Pediatr. Res. 82, 1030–1038 (2017).
Paton, M. adisonC. B. et al. Human umbilical cord blood therapy protects cerebral white matter from systemic Lps exposure in preterm fetal sheep. Dev. Neurosci. 40, 258–270 (2018).
Xi, Y., Yue, G., Gao, S., Ju, R. & Wang, Y. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. Stem Cell Res. Ther. 13, 1–458 (2022).
Penny, T. R. et al. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. 1746, 147001 (2020).
Aridas, J. D. S. et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. J. Physiol. 594, 1421–1435 (2016).
Penny, T. R. et al. Human umbilical cord therapy improves long-term behavioral outcomes following neonatal hypoxic ischemic brain injury. Front. Physiol. 10, 283–283 (2019).
Wong, A., Yuen, P. M. P., Li, K., Yu, A. L. M. & Tsoi, W. C. Cord Blood collection before and after placental delivery : levels of nucleated cells, haematopoietic progenitor cells, leukocyte subpopulations and macroscopic clots. Bone Marrow Transplant. 27, 133–138 (2001).
Broxmeyer, H. E. et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl. Acad. Sci. 86, 3828–3832 (1989).
Gluckman, E. et al. Outcome of cord-blood transplantation from related and unrelated donors. N. Engl. J. Med. 337, 373–381 (1997).
Zhou, L. et al. Feasibility of cord blood collection for autologous cell therapy applications in extremely preterm infants. Cytotherapy 25, 458–462 (2023).
Cotten, C. M. et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J. Pediatr. 2014;164:973–979.e
Tsuji, M. et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Sci. Rep. 10, 4603–4603 (2020).
Zhou, L. et al. Umbilical cord blood and cord tissue-derived cell therapies for neonatal morbidities: current status and future challenges. Stem Cells Transl. Med 11, 135–145 (2022).
Nagamura-Inoue, T. & Nagamura, F. Umbilical cord blood and cord tissue banking as somatic stem cell resources to support medical cell modalities. Inflamm. Regen. 43, 59 (2023).
Lasky, L. C. et al. In utero or ex utero cord blood collection: Which is better?. Transfusion 42, 1261–1267 (2002).
Solves, P. et al. Comparison between two strategies for umbilical cord blood collection. Bone Marrow Transpl. 31, 269–273 (2003).
Hare, J. et al. Optimal umbilical cord blood collection, processing and cryopreservation methods for sustained public cord blood banking. Cytotherapy 23, 1029–1035 (2021).
Katheria, A. C. Umbilical cord milking: a review. Front. Pediatr. 6, 335 (2018).
Katheria, A. C. et al. Stem cell composition of umbilical cord blood following milking compared with delayed clamping of the cord appears better suited for promoting hematopoiesis. J. Pediatr. 216, 222–226 (2020).
Katheria, A. C. et al. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am. J. Obstet. Gynecol. 228, 217.e211–217.e214 (2023).
McAdams, R. M., Fay, E. & Delaney, S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J. Perinatol. 38, 245–250 (2018).
Koo, J., Kilicdag, H. & Katheria, A. Umbilical cord milking-benefits and risks. Front. Pediatr. 11, 1146057–1146057 (2023).
Bassiouny, M. R., El-Chennawi, F., Mansour, A. K., Yahia, S. & Darwish, A. Optimal method for collection of umbilical cord blood: an Egyptian trial for a public cord blood bank. Transfusion 55, 1263–1268 (2015).
Fumarola, S. A. L. A. A. L. G. A. P. L. A. P. L. Predictors of cord blood unit cell content in a volume unrestricted large series collections: a chance for a fast and cheap multiparameter selection model. Stem Cell Res. Ther. 13, 246–246 (2022).
Gonzalez-Ryan, L. A. V. S. K. A. C. K. D. A. G. N. Umbilical cord blood banking: procedural and ethical concerns for this new birth option. Pediatr. Nurs. 26, 105–110 (2000).
Wong, A., Yuen, P. M., Li, K., Yu, A. L. & Tsoi, W. C. Cord blood collection before and after placental delivery: levels of nucleated cells, haematopoietic progenitor cells, leukocyte subpopulations and macroscopic clots. Bone Marrow Transpl. 27, 133–138 (2001).
Patra, A., Huang, H., Bauer, J. A. & Giannone, P. J. Neurological consequences of systemic inflammation in the premature neonate. Neural Regen. Res. 12, 890–896 (2017).
Geiger, R., Ellemunter, H., Fink, F. M., Falk, M. & Tilg, H. Circulating interleukin-1 receptor antagonist levels in neonates. Eur. J. Pediatr. 155, 811–814 (1996).
Arend, W. P. The Balance between Il-1 and Il-1ra in Disease. Cytokine Growth Factor Rev. 13, 323–340 (2002).
Dewberry, R., Holden, H., Crossman, D. & Francis, S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20, 2394–2400 (2000).
Ferrara, N., Gerber, H.-P. & LeCouter, J. The biology of vegf and its receptors. Nat. Med. 9, 669–676 (2003).
Asahara, T. et al. Vegf contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial Progenitor Cells. EMBO J. 18, 3964–3972 (1999).
Katheria, A. C., Truong, G., Cousins, L., Oshiro, B. & Finer, N. N. Umbilical cord milking versus delayed cord clamping in preterm infants. Obstet. Gynecol. Surv. 70, 683–684 (2015).
Funding
No specific funding was secured for this research. R.A.S.M. and A.R. are supported by PhD Scholarships from Monash University. A.R. receives funding from Lions Cord Blood Foundation and Royal Australasian College of Physicians. A.M. and C.A.M. receive funding for their research from the National Health and Medical Research Council of Australia. T.R.P., A.M., and C.A.M. receive funding from the Medical Research Future Fund. A.M. also receives funding from Monash Health Foundation, National Stem Cell Foundation of Australia, Lions Cord Blood Foundation and Cerebral Palsy Alliance.
Author information
Authors and Affiliations
Contributions
R.A.S.M.: Conduct of experiments, data collection and analysis, interpretation, manuscript writing. K.C.: recruitment, conduct of experiments, data collection, manuscript editing. A.R.: recruitment, conduct of experiments, data collection and analysis, manuscript editing. L.Z.: recruitment, conduct of experiments, data collection, manuscript editing. T.R.P.: conception and design, conduct of experiments, data analysis, manuscript editing, supervision. C.A.M.: conception and design, conduct of experiments, data analysis and interpretation, manuscript editing, supervision. A.M.: conception and design, data analysis and interpretation, manuscript editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Patient consent was obtained as part of an ongoing ethics application as stated in the methods.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marzan, R.A.S., Connelly, K., Razak, A. et al. Umbilical cord milking as a technique to harvest cord blood derived cells for regenerative applications. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04687-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04687-6