Abstract
Background
Perinatal disruption of sodium homeostasis, which is critical for organ and cell function, may impact growth, metabolism, and pulmonary function.
Methods
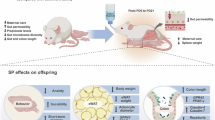
Two murine models were studied. First, maternal mice were supplied with a standard (0.15%) or low sodium (0.04% Na) diet from embryonic day 18 until postnatal day 21 (E18-P21). Second, offspring of mothers on standard Na were administered daily furosemide (30 mg/kg ip) on P10-P13 or sham injection. All pups received 0.15% Na diet at weaning. In male offspring, weight and body composition were serially measured while total energy expenditure was determined at 8–9 weeks of age. Ventilatory function was assessed at 3–5 weeks and again at 6–8 weeks of age in males and females. Lung structure was assessed at 9–10 weeks.
Results
Maternal low Na diet programmed significantly decreased weight gain in offspring associated with increased total energy expenditure. No significant effects on lung structure or breathing were seen. Furosemide resulted in increased weight, fat and fat-free mass in males. Furosemide was also associated with significantly decreased minute ventilation and tidal volume in males without changes to lung structure.
Conclusion
Perinatal Na homeostasis is crucial for long-term growth, metabolism, and pulmonary function.
Impact
-
Maintenance of early life sodium homeostasis is essential for growth and organ development
-
Using different mouse models, we demonstrated a crucial role of early Na balance in long term growth, body composition, and metabolic and respiratory functions.
-
Optimized intervention to maintain sodium homeostasis may improve long-term outcomes of preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author to researchers who provide a methodologically sound proposal. Data requestors will need to sign a data use agreement approved by the Medical College of Wisconsin.
References
Burnier, M. (ed.). Sodium in Health and Disease 1st ed (CRC Press, 2007)
Wang, Y. J., Yeh, T. L., Shih, M. C., Tu, Y. K. & Chien, K. L. Dietary sodium intake and risk of cardiovascular disease: a systematic review and dose-response meta-analysis. Nutrients 12, 2934 (2020).
Diringer, M. Neurologic manifestations of major electrolyte abnormalities. Handb. Clin. Neurol. 141, 705–713 (2017).
Sakuyama, H. et al. Influence of gestational salt restriction in fetal growth and in development of diseases in adulthood. J. Biomed. Sci. 23, 12 (2016).
Isemann, B., Mueller, E. W., Narendran, V. & Akinbi, H. Impact of early sodium supplementation on hyponatremia and growth in premature infants: a randomized controlled trial. J. Parenter. Enter. Nutr. 40, 342–9 (2016).
Stalter, E. J. et al. Somatic growth outcomes in response to an individualized neonatal sodium supplementation protocol. J. Perinatol. 45, 305–311 (2025).
Petersen, R. Y., Clermont, D., Williams, H. L., Buchanan, P. & Hillman, N. H. Oral sodium supplementation on growth and hypertension in preterm infants: an observational cohort study. J. Perinatol. 44, 1515–1522 (2024).
Bamat, N. A. et al. Loop diuretics in severe bronchopulmonary dysplasia: cumulative use and associations with mortality and age at discharge. J. Pediatr. 231, 43–49.e3 (2021).
Tan, C., Sehgal, K., Sehgal, K., Krishnappa, S. B. & Sehgal, A. Diuretic use in infants with developing or established chronic lung disease: a practice looking for evidence. J. Paediatr. Child Health 56, 1189–1193 (2020).
Segar, J. L. et al. Dissociable effects of dietary sodium in early life upon somatic growth, fluid homeostasis, and spatial memory in mice of both sexes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 320, R438–R451 (2021).
Ziegler, A. A. et al. Early-life sodium deprivation programs long-term changes in ingestive behaviors and energy expenditure in C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 325, R576–R592 (2023).
Ziegler, A. A. et al. Early-life sodium restriction programs autonomic dysfunction and salt sensitivity in male C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 328, R109–R120 (2025).
Vidonho, A. F. et al. Perinatal salt restriction: a new pathway to programming insulin resistance and dyslipidemia in adult Wistar rats. Pediatr. Res 56, 842–8 (2004).
Bursey, R. G. & Watson, M. L. The effect of sodium restriction during gestation of offspring brain development in rats. Am. J. Clin. Nutr. 37, 43–51 (1983).
Chou, R. et al. Low-salt intake during mating or gestation in rats is associated with low birth and survival rates of babies. J. Nutr. Metab. 2014, 212089 (2014).
Mangold, J. E. & Hill, D. L. Extensive reorganization of primary afferent projections into the gustatory brainstem induced by feeding a sodium-restricted diet during development: less is more. J. Neurosci. 27, 4650–62 (2007).
Siqueira, F. R., Furukawa, L. N., Oliveira, I. B. & Heimann, J. C. Glucose metabolism and hepatic Igf1 DNA methylation are altered in the offspring of dams fed a low-salt diet during pregnancy. Physiol. Behav. 154, 68–75 (2016).
Perillán, C., Núñez, P., Costales, M., Vijande, M. & Argüelles, J. Ingestive behavior in rat pups is modified by maternal sodium depletion. Psicothema 24, 422–6 (2012).
Wassner, S. J. Altered growth and protein turnover in rats fed sodium-deficient diets. Pediatr. Res 26, 608–13 (1989).
Gallaher, K. J., Wolpert, E., Wassner, S. & Rannels, D. E. Effect of diet-induced sodium deficiency on normal and compensatory growth of the lung in young rats. Pediatr. Res. 28, 455–9 (1990).
Matalon, S., Bartoszewski, R. & Collawn, J. F. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1229–38 (2015).
Iwamoto, L. M., Fujiwara, N., Nakamura, K. T. & Wada, R. K. Na-K-2Cl cotransporter inhibition impairs human lung cellular proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L510–4 (2004).
Klein, J. D., Perry, P. B. & O’Neill, W. C. Regulation by cell volume of Na(+)-K(+)-2Cl- cotransport in vascular endothelial cells: role of protein phosphorylation. J. Membr. Biol. 132, 243–52 (1993).
Ehrenkranz, R. A. et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr. Res 69, 522–9 (2011).
Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals 8th edn (National Academies Press, 2011).
Warburton, D. et al. Lung organogenesis. Curr. Top. Dev. Biol. 90, 73–158 (2010).
Caravagna, C. et al. Prenatal hypoxia induces Cl- cotransporters KCC2 and NKCC1 developmental abnormality and disturbs the influence of GABAA and glycine receptors on fictive breathing in a newborn rat. Front. Physiol. 13, 786714 (2022).
Xue, J., Thomas, L., Dominguez Rieg, J. A., Fenton, R. A. & Rieg, T. NHE3 in the thick ascending limb is required for sustained but not acute furosemide-induced urinary acidification. Am. J. Physiol. Ren. Physiol. 323, F141–F155 (2022).
Al Za’abi, M. et al. The effects of furosemide on behavioral and hormonal parameters in male and female mice subjected to immobilization and cold-water stress. J. Exp. Pharm. 13, 637–643 (2021).
Reho, J. J. et al. Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front. Physiol. 13, 855054 (2022).
Weir, J. B. de V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9 (1949).
Mouradian, G. C., Kilby, M., Alvarez, S., Kaplan, K. & Hodges, M. R. Mortality and ventilatory effects of central serotonin deficiency during postnatal development depend on age but not sex. Physiol. Rep. 9, e14946 (2021).
Prada-Dacasa, P., Urpi, A., Sánchez-Benito, L., Bianchi, P. & Quintana, A. Measuring breathing patterns in mice using whole-body plethysmography. Bio Protoc. 10, e3741 (2020).
Lim, R. et al. Measuring respiratory function in mice using unrestrained whole-body plethysmography. J. Vis. Exp. 51755 (2014).
Davenport, M. L., Sherrill, T. P., Blackwell, T. S. & Edmonds, M. D. Perfusion and inflation of the mouse lung for tumor histology. J. Vis. Exp. https://doi.org/10.3791/60605 (2020).
Braber, S., Verheijden, K.aT., Henricks, P.aJ., Kraneveld, A. D. & Folkerts, G. A comparison of fixation methods on lung morphology in a murine model of emphysema. Am. J. Physiol. Lung Cell. Mol. Physiol. 299, L843–51 (2010).
Hsia, C. C. W., Hyde, D. M., Ochs, M. & Weibel, E. R. An Official Research Policy Statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 181, 394–418 (2010).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–82 (2012).
Segar, J. L. Renal adaptive changes and sodium handling in the fetal-to-newborn transition. Semin. Fetal Neonatal Med. 22, 76–82 (2017).
Segar, D. E. et al. Physiological approach to sodium supplementation in preterm infants. Am. J. Perinatol. 35, 994–1000 (2018).
Kim, Y. J. et al. Risk factors for late-onset hyponatremia and its influence on neonatal outcomes in preterm infants. J. Korean Med. Sci. 30, 456–62 (2015).
Bouret, S. G. Developmental programming of hypothalamic melanocortin circuits. Exp. Mol. Med. 54, 403–413, https://doi.org/10.1038/s12276-021-00625-8 (2022).
Bouret, S. G. Development of hypothalamic circuits that control food intake and energy balance. In. Appetite and Food Intake: Central Control (ed. Harris, R. B. S.) 135–154 (CRC Press/Taylor & Francis, 2017).
Nilsson, I., Johansen, J. E., Schalling, M., Hokfelt, T. & Fetissov, S. O. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res. Dev. Brain Res. 155, 147–154 (2005).
Pozarska, A. et al. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L882–L895 (2017).
Segar, J. L., Grobe, C. C. & Grobe, J. L. Fetal storage of osmotically inactive sodium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R512–R514 (2020).
Schafflhuber, M. et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am. J. Physiol. Ren. Physiol. 292, F1490–500 (2007).
Deng, G. & Grobe, J. L. The renin-angiotensin system in the arcuate nucleus controls resting metabolic rate. Curr. Opin. Nephrol. Hypertens. 28, 120–127 (2019).
Lawton, S. B. et al. Angiotensin in the arcuate: mechanisms integrating cardiometabolic control: the 2022 COH mid-career award for research excellence. Hypertension 81, 2209–2217 (2024).
Harding, R. & De Matteo, R. The influence of nutrition on lung development before and after birth. In The Lung 2nd Ed (eds Harding, R. & Pinkerton, K. E.). 349–368 (Academic Press, 2014)
Pivovarov, A. S., Calahorro, F. & Walker, R. J. Na+/K+-pump and neurotransmitter membrane receptors. Invert. Neurosci. 19, 1 (2018).
Shi, Y. et al. Nalcn Is a “Leak” sodium channel that regulates excitability of brainstem chemosensory neurons and breathing. J. Neurosci. 36, 8174–87 (2016).
Putnam, R. W., Filosa, J. A. & Ritucci, N. A. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am. J. Physiol. Cell Physiol. 287, C1493–526 (2004).
Donnelly, D. F. Voltage-gated Na(+) channels in chemoreceptor afferent neurons-potential roles and changes with development. Respir. Physiol. Neurobiol. 185, 67–74 (2013).
Pacifici, G. M. Clinical pharmacology of furosemide in neonates: a review. Pharmaceuticals 6, 1094–129 (2013).
Marcoux, A. A. et al. Molecular characteristics and physiological roles of Na+ -K+ -Cl- cotransporter 2. J. Cell Physiol. 236, 1712–1729 (2021).
Virtanen, M. A., Uvarov, P., Hübner, C. A. & Kaila, K. NKCC1, an elusive molecular target in brain development: making sense of the existing data. Cells 9, 2607 (2020).
Chamma, I., Chevy, Q., Poncer, J. C. & Lévi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 6, 5 (2012).
Peerboom, C. & Wierenga, C. J. The postnatal GABA shift: a developmental perspective. Neurosci. Biobehav. Rev. 124, 179–192 (2021).
Kaffman, A. & Meaney, M. J. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J. Child Psychol. Psychiatry 48, 224–44 (2007).
Lawton, S. B. et al. Differences in fluid, electrolyte, and energy balance in C57BL/6J mice (Mus musculus) in metabolic caging at thermoneutral or standard room temperatures. J. Am. Assoc. Lab. Anim. Sci. 63, 190–200 (2024).
Acknowledgements
The authors gratefully acknowledge assistance provided by the Medical College of Wisconsin Comprehensive Rodent Metabolic Phenotyping Core and Biomedical Resource Center. The authors also wish to acknowledge technical support from Kelsey K. Wackman and Ko-Ting Lu. Schematics were made using Biorender.com.
Funding
This work was supported by the NIH grants DK133121, HL134850, the Medical College of Wisconsin Clinical and Translational Science Institute (UL1TR001436), the Children’s Wisconsin Children’s Research Institute, endowments from the Butenhoff and Mellowes families, and the Advancing a Healthier Wisconsin Endowment to the Medical College of Wisconsin.
Author information
Authors and Affiliations
Contributions
A.M., B.A., J.S., and G.M. conceived the study. A.M., B.A. and C.G. performed the experiments. A.M., B.A., J.G., J.S., and G.M. analyzed the data. J.G. and G. M. provided essential input. A.M, B.A, and J.S. wrote the manuscript. All authors critically reviewed and revised the manuscript and gave their final approval for manuscript submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madison, A.M., Araya, B.R., Grobe, C.C. et al. Maternal low sodium intake and early postnatal diuretics program metabolic and ventilatory dysfunction in mice. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04689-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04689-4