Abstract
Background
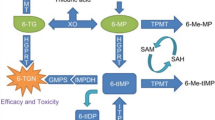
Children with acute lymphoblastic leukemia (ALL) may develop hypoglycemia, potentially attributable to mercaptopurine (6-MP) during long-term maintenance chemotherapy. Since hypoglycemia is harmful to childhood neurodevelopment, it is necessary to examine its occurrence during chemotherapy with and without 6-MP beyond the maintenance stage for ALL, along with the risk factors.
Methods
ALL patients received CAM-1 regimen (cyclophosphamide, cytarabine, and 6-MP). 6-MP was randomized to conventional administration at night before bedtime (Group A) or in the afternoon between lunch and dinner (Group B). Children with acute myeloid leukemia received non-6MP-containing chemotherapy (Group C).
Results
Group C showed no hypoglycemia. Among patients on CAM-1, 33% developed hypoglycemia, 48% of whom were symptomatic. There was no significant difference in hypoglycemic incidence (P = 0.933) between Groups A and B. No further hypoglycemic episodes were observed after shortening overnight fasting period in most cases. Multivariate analysis identified young age, higher serum bilirubin levels, and longer overnight fasting as significant risk factors for hypoglycemia in children receiving 6-MP.
Conclusion
Hypoglycemia is also prevalent in children exposed to short-term 6-MP but not in those without such exposure. Shortening overnight fasting period, rather than changing 6-MP schedule, is more critical in preventing hypoglycemia in young children.
Impact
-
This study reveals that hypoglycemia occurs frequently in children with short-term exposure to 6-MP, as documented for long-term exposure in published literature.
-
The data demonstrate that it is 6-MP that is directly associated with hypoglycemia.
-
Prolonged durations of overnight fasting, especially in younger individuals with hepatotoxicity, constitute a risk factor for developing hypoglycemia.
-
Shortening the overnight fasting period, rather than changing the 6-MP schedule, is critical to prevent hypoglycemia and adverse neurodevelopment in children, even if they are receiving short-term 6-MP treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data are available on request from the authors.
References
Worth, C. et al. Continuous glucose monitoring for children with hypoglycaemia: evidence in 2023. Front. Endocrinol. 14, 1116864 (2023).
Avatapalle, H. B. et al. Abnormal neurodevelopmental outcomes are common in children with transient congenital hyperinsulinism. Front. Endocrinol. 4, 60 (2013).
Albiroty, K. & Sabahi, A. A. Severe recurrent nocturnal hypoglycemia during chemotherapy with 6-mercaptopurine in 2 children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 45, 162–163 (2023).
Cho, E. M., Moon, J. E., Lee, S. J. & Ko, C. W. Severe recurrent nocturnal hypoglycemia during chemotherapy with 6-mercaptopurine in a child with acute lymphoblastic leukemia. Ann. Pediatr. Endocrinol. Metab. 23, 226–228 (2018).
Melachuri, S., Gandrud, L. & Bostrom, B. The association between fasting hypoglycemia and methylated mercaptopurine metabolites in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer. 61, 1003–1006 (2014).
Miller, M. B., Brackett, J., Schafer, E. S. & Rau, R. E. Prevention of mercaptopurine induced hypoglycemia using allopurinol to reduce methylated thiopurine metabolites. Pediatr. Blood Cancer 66, e27577 (2019).
Bay, A. et al. Symptomatic hypoglycemia: an unusual side effect of oral purine analogues for treatment of ALL. Pediatr. Blood Cancer. 47, 330–331 (2006).
Jiang, M. & Ahmet, A. Hypoglycemia during treatment of acute lymphoblastic leukemia. Paediatr. Child Health. 28, 305–306 (2023).
El-Bitar, M. K., Muwakkit, S. A. & Dabbagh, O. Severe hypoglycemic seizures in a child receiving 6-mercaptopurine. J. Pediatr. Hematol. Oncol. 33, e75–e76 (2011).
Trelinska, J. et al. Hypoglycemia and glycemic variability among children with acute lymphoblastic leukemia during maintenance therapy. Leuk. Lymphoma 52, 1704–1710 (2011).
Halonen, P., Salo, M. K. & Mäkipernaa, A. Fasting hypoglycemia is common during maintenance therapy for childhood acute lymphoblastic leukemia. J. Pediatr. 138, 428–431 (2001).
Ziino, O. et al. Symptomatic hypoglycemia in children receiving oral purine analogues for treatment of childhood Acute lymphoblastic leukemia. Med. Pediatr. Oncol. 39, 32–34 (2002).
Aricó, M. et al. The seventh international childhood acute lymphoblastic leukemia workshop report: Palermo, Italy, January 29-30, 2005. Leukemia 19, 1145–1152 (2005).
Landier, W. et al. Mercaptopurine ingestion habits, red cell thioguanine nucleotide levels, and relapse risk in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group Study AALL03N1. J. Clin. Oncol. 35, 1730–1736 (2017).
Samis, J. et al. Recognizing endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic myelogenous leukemia. Pediatr. Blood Cancer 63, 1332–1338 (2016).
Faustino, E. V., Hirshberg, E. L. & Bogue, C. W. Hypoglycemia in critically ill children. J. Diabetes Sci. Technol. 6, 48–57 (2012).
Li, H., Ji, C. Y., Zong, X. N. & Zhang, Y. Q. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Chin J. Pediatr. 47, 493–498 (2009).
Rosenfeld, E. et al. Incidence and risk factors for hypoglycemia during maintenance chemotherapy in pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 69, e29467 (2022).
Mary, R. J. et al. Hypoglycemia Associated With PEG-asparaginase and 6-MP Therapy During Treatment of Acute Lymphoblastic Leukemia in Pediatric Patients: A Case Series. J. Pediatr. Hematol. Oncol. 46, e121–e126 (2024).
Supandi, S., Harahap, Y., Harmita, H. & Andalusia, R. Quantification of 6-Mercaptopurine and its metabolites in patients with acute lymphoblastic leukemia using dried blood spots and UPLC-MS/MS. Sci. Pharm. 86, 18 (2018).
Marinaki, A. M. & Arenas-Hernandez, M. Reducing risk in thiopurine therapy. Xenobiotica. 50, 101–109 (2020).
Giamanco, N. M. et al. Allopurinol use during maintenance therapy for acute lymphoblastic leukemia avoids mercaptopurine-related hepatotoxicity. J. Pediatr. Hematol. Oncol. 38, 147–151 (2016).
Barone, T., Dandekar, S., McKeone, D. & Mulieri, K. Assessment on the use of allopurinol to improve safety and efficacy of mercaptopurine in pediatric patients with Acute Lymphoblastic Leukemia and Lymphoma during maintenance therapy. Cancer Rep. 7, e1987 (2024).
Zhang, M. & Bostrom, B. Allopurinol reverses mercaptopurine-induced hypoglycemia in patients with acute lymphoblastic leukemia. F1000Res. 8, 176 (2019).
Hoe, F. M. Hypoglycemia in infants and children. Adv. Pediatr. 55, 367–384 (2008).
Mohamed, A. et al. Reducing morning hypoglycemia among children undergoing treatment for acute lymphoblastic leukemia. JCO Oncol. Pract. 17, e901–e907 (2021).
Acknowledgements
We thank the patients and their families for their willingness to participate in this trial, and all participants and research staff.
Funding
This work was supported by grants in China from the Medical Science and Technology Research Foundations of Guangdong Province, China (A2022443 and A2024559), and the Science and Technology Planning Project of Huizhou, China (2022CZ010007).
Author information
Authors and Affiliations
Contributions
Conception and design: Zhang X.L., Huang L.B., Tang Y.L. and Luo X.Q. Generating the random allocation sequence, assigning participants to interventions: Chen Z.Y. and Zhang X.L. Enrolling participants: Zhang X.L., Huang L.B., and Tang Y.L. Collection and assembly of data: Chen Z.Y., Li Q.R., Liao L.H., Chen X.L., Xiao X.L., Jin H., Li Y., Wang L.N., Liang C., Fan Z., Yue T.F., and Yang C.Y. Data analysis and interpretation: Zhang X.L., Huang L.B. and Tang Y.L. Statistical analysis: Zhang X.L., Huang L.B., Tang Y.L., Chen Z.Y., Li Q.R. and Liao L.H. Manuscript writing and final approval of manuscript: all authors. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent was obtained from the parents of the study participants before enrollment in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, ZY., Li, QR., Liao, L. et al. Impact of mercaptopurine schedule on hypoglycemia in leukemic children: randomized trial and risk factor analysis. Pediatr Res (2026). https://doi.org/10.1038/s41390-025-04728-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04728-0