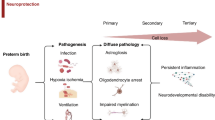
Abstract
Critically ill preterm and term neonates are uniquely vulnerable to and constantly confront potential harmful and unintended exposures in every aspect of their care, including diagnostic imaging, sensory environments, medications, nutrition, blood products, and device exposures. The rapidly changing pathology, physiology, and metabolism of these infants, along with a lack of treatments tailored to the needs of neonates, lead to unintended negative consequences with impacts reaching far out into adulthood. Families, nurses, clinicians, and researchers provide the best care for neonates with the resources and knowledge available, but more needs to be done from the healthcare policy and societal levels. More research is needed to understand the negative impacts of environmental exposures on neonates and children in general. Concerted efforts should focus on eliminating known toxic and harmful substances from commercial products used in neonatal care, and alternatives should be made available. Resource allocation is needed by community leaders and health policy makers, through regulations and incentives, to ensure that neonates and children can have healthy, happy, and productive lives. Our society should be judged by how we care for and treat this most vulnerable population, who deserve environments and treatments free from unintended, unnecessary harmful exposures.
Impact
-
Technological advances have significantly improved survival of critically ill term and pre-term infants, but pose a unique challenge of exposure to multiple environmental toxins.
-
In this review, we have summarized these exposures and the pathways through which they may negatively impact the neurodevelopmental outcomes in this highly vulnerable population.
-
Ongoing environmental exposures in the NICU are a global healthcare problem and need policies and resources in place to mitigate their negative impact on infant and child health outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stark, A. et al. Medication use in the neonatal intensive care unit and changes from 2010 to 2018. J. Pediatr. 240, 66–71.e64 (2022).
Vachharajani, A., Vachharajani, N. A. & Najaf, T. Neonatal Radiation Exposure. NeoReviews 14, e190–e197 (2013).
Berlin, S. in Principles of Neonatology (Maheshwari, A. ed.) 33–36 (Elsevier, 2024).
Brody, A. S., Frush, D. P., Huda, W. & Brent, R. L. Radiation Risk to Children from Computed Tomography. Pediatrics 120, 677–682 (2007).
Edison, P. et al. Reducing radiation hazard opportunities in neonatal unit: quality improvement in radiation safety practices. BMJ Open Qual. 6, e000128 (2017).
Bhattacharjee, I., Volpe, M., Bhattacharya, S., Sibley, C. & Church, P. Neurodevelopmental impact of early diagnostic imaging in preterm infants: quantifying risk and the role of point-of-care ultrasound. Front. Pediatr. 13, 1642629 (2025).
Gislason-Lee, A. J. Patient X-Ray Exposure and Alara in the Neonatal Intensive Care Unit: Global Patterns. Pediatr. Neonatol. 62, 3–10 (2021).
Makri, T. et al. Radiation Risk Assessment in Neonatal Radiographic Examinations of the Chest and Abdomen: A Clinical and Monte Carlo Dosimetry Study. Phys. Med. Biol. 51, 5023–5033 (2006).
Kartikeswar, G. A. P., Parikh, T. B., Pandya, D. & Pandit, A. Ionizing Radiation Exposure in Nicu. Indian J. Pediatr. 87, 158–160 (2020).
Kutanzi, K. R., Lumen, A., Koturbash, I. & Miousse, I. R. Pediatric Exposures to Ionizing Radiation: Carcinogenic Considerations. Int. J. Environ. Res. Public Health 13 (2016).
Vol. 2 (Radiation, U. N. S. C. O. T. E. O. A. ed. (United Nations, 2008).
Wilson-Costello, D., Rao, P. S., Morrison, S. & Hack, M. Radiation Exposure from Diagnostic Radiographs in Extremely Low Birth Weight Infants. Pediatrics 97, 369–374 (1996).
Frush, D. in AAP News (American Academy of Pediatrics, 2020).
Seibert, J. A. Digital Radiography: Image Quality and Radiation Dose. Health Phys. 95, 586–598 (2008).
Longo, M. et al. Quantification of Scatter Radiation from Radiographic Procedures in a Neonatal Intensive Care Unit. Pediatr. Radio. 48, 715–721 (2018).
Khan, I. & Khan, M. A. B. in Statpearls (StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC., 2025).
Mayhew, K. J., Lawrence, S. L., Squires, J. E. & Harrison, D. Elevated Sound Levels in the Neonatal Intensive Care Unit: What Is Causing the Problem? Adv. Neonatal Care 22, E207–e216 (2022).
White, R. D. Recommended Standards for Newborn Icu Design, 9th Edition. J. Perinatol. 40, 2–4 (2020).
Balk, S. J., Bochner, R. E., Ramdhanie, M. A. & Reilly, B. K. Preventing Excessive Noise Exposure in Infants, Children, and Adolescents. Pediatrics 152, e2023063752 (2023).
Pati, S. et al. Chronic Postnatal Chemogenetic Activation of Forebrain Excitatory Neurons Evokes Persistent Changes in Mood Behavior. Elife 9, e56171 (2020).
de Paula Machado, A. C. C., de Castro Magalhães, L., de Oliveira, S. R. & Bouzada, M. C. F. Is Sensory Processing Associated with Prematurity, Motor and Cognitive Development at 12 Months of Age? Early Hum. Dev. 139, 104852 (2019).
Lickliter, R. The Integrated Development of Sensory Organization. Clin. Perinatol. 38, 591–603 (2011).
Smith, G. C. et al. Neonatal Intensive Care Unit Stress Is Associated with Brain Development in Preterm Infants. Ann. Neurol. 70, 541–549 (2011).
Ryckman, J., Hilton, C., Rogers, C. & Pineda, R. Sensory Processing Disorder in Preterm Infants During Early Childhood and Relationships to Early Neurobehavior. Early Hum. Dev. 113, 18–22 (2017).
Lejeune, F. et al. Sound Interferes with the Early Tactile Manual Abilities of Preterm Infants. Sci. Rep. 6, 23329 (2016).
Almadhoob, A. & Ohlsson, A. Sound Reduction Management in the Neonatal Intensive Care Unit for Preterm or Very Low Birth Weight Infants. Cochrane Database Syst. Rev. 1, Cd010333 (2020).
Taheri, L., Jahromi, M. K., Abbasi, M. & Hojat, M. Effect of Recorded Male Lullaby on Physiologic Response of Neonates in NICU. Appl Nurs. Res. 33, 127–130 (2017).
Neri, E. et al. Parental Book-Reading to Preterm Born Infants in NICU: The Effects on Language Development in the First Two Years. Int. J. Environ. Res. Public Health 18, 11361 (2021).
Boissel, L. et al. A Narrative Review of the Effect of Parent-Child Shared Reading in Preterm Infants. Front. Pediatr. 10, 860391 (2022).
Cevasco-Trotter, A. M., Hamm, E. L., Yang, X. & Parton, J. Multimodal Neurological Enhancement Intervention for Self-Regulation in Premature Infants. Adv. Neonatal Care 19, E3–e11 (2019).
Teles, L. A., Arcanjo, F. P. N., Cardoso, K. M. & Justino, J. S. Impact of skin-to-skin contact on acute procedural pain in newborns: a systematic review and meta-analysis. J. Pediatr. 101, 101442 (2025).
La Rosa, V. L., Geraci, A., Iacono, A. & Commodari, E. Affective Touch in Preterm Infant Development: Neurobiological Mechanisms and Implications for Child-Caregiver Attachment and Neonatal Care. Children (Basel) 11, 1407 (2024).
Séassau, A., Munos, P., Gire, C., Tosello, B. & Carchon, I. Neonatal Care Unit Interventions on Preterm Development. Children (Basel) 10, 999 (2023).
Nist, M. D., Cistone, N. & Pickler, R. H. Improving Outcomes for Preterm Infants: Mitigating Stress Exposure. Semin. Perinatol. 152153 (2025). Epub ahead of print.
Altimier, L. & Phillips, R. The Neonatal Integrative Developmental Care Model: Advanced Clinical Applications of the Seven Core Measures for Neuroprotective Family-Centered Developmental Care. Newborn Infant Nurs. Rev. 16, 230–244 (2016).
Purpura, G. et al. Effects of Social and Sensory Deprivation in Newborns: A Lesson from the Covid-19 Experience. Early Hum. Dev. 185, 105853 (2023).
El-Atawi, K., Alallah, J., Al Qurashi, M. & Saleh, M. Impact of Single-Family Room Care on Neonatal Outcomes: A Systematic Review. Cureus 17, e88499 (2025).
van Veenendaal, N. R. et al. Hospitalising Preterm Infants in Single Family Rooms Versus Open Bay Units: A Systematic Review and Meta-Analysis of Impact on Parents. EClinicalMedicine 23, 100388 (2020).
O’Callaghan, N., Dee, A. & Philip, R. K. Evidence-Based Design for Neonatal Units: A Systematic Review. Matern Health Neonatol. Perinatol. 5, 6 (2019).
Pineda, R. G. et al. Alterations in Brain Structure and Neurodevelopmental Outcome in Preterm Infants Hospitalized in Different Neonatal Intensive Care Unit Environments. J. Pediatr. 164, 52–60.e52 (2014).
Robertson, A. F. Reflections on Errors in Neonatology Iii. The “Experienced” Years, 1970 to 2000. J. Perinatol. 23, 240–249 (2003).
O’Brien, F. et al. Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine. Int. J. Mol. Sci. 20, 2688 (2019).
Whittaker, A. et al. Toxic Additives in Medication for Preterm Infants. Arch. Dis. Child Fetal Neonatal Ed. 94, F236–F240 (2009).
Akinmboni, T. O., Davis, N. L., Falck, A. J., Bearer, C. F. & Mooney, S. M. Excipient Exposure in Very Low Birth Weight Preterm Neonates. J. Perinatol. 38, 169–174 (2018).
Chung, E., Reinaker, K. & Meyers, R. Ethanol Content of Medications and Its Effect on Blood Alcohol Concentration in Pediatric Patients. J. Pediatr. Pharm. Ther. 29, 188–194 (2024).
Johnson, J., Akinboyo, I. C. & Schaffzin, J. K. Infection Prevention in the Neonatal Intensive Care Unit. Clin. Perinatol. 48, 413–429 (2021).
Polin, R. A. et al. Strategies for Prevention of Health Care–Associated Infections in the Nicu. Pediatrics 129, e1085–e1093 (2012).
Muhd Helmi, M. A., Lai, N. M., Van Rostenberghe, H., Ayub, I. & Mading, E. Antiseptic Solutions for Skin Preparation During Central Catheter Insertion in Neonates. Cochrane Database Syst. Rev. 5, Cd013841 (2023).
Chapman, A. K. et al. Absorption and Tolerability of Aqueous Chlorhexidine Gluconate Used for Skin Antisepsis Prior to Catheter Insertion in Preterm Neonates. J. Perinatol. 33, 768–771 (2013).
Kieran, E. A. et al. 2% Chlorhexidine-70% Isopropyl Alcohol Versus 10% Povidone-Iodine for Insertion Site Cleaning before Central Line Insertion in Preterm Infants: A Randomised Trial. Arch. Dis. Child Fetal Neonatal Ed. 103, F101–f106 (2018).
Devlin, L. A. et al. Neonatal Opioid Withdrawal Syndrome: A Review of the Science and a Look toward the Use of Buprenorphine for Affected Infants. J. Perinatol. 42, 300–306 (2022).
Yilmaz, B., Terekeci, H., Sandal, S. & Kelestimur, F. Endocrine Disrupting Chemicals: Exposure, Effects on Human Health, Mechanism of Action, Models for Testing and Strategies for Prevention. Rev. Endocr. Metab. Disord. 21, 127–147 (2020).
Demeneix, B. A. How Fossil Fuel-Derived Pesticides and Plastics Harm Health, Biodiversity, and the Climate. Lancet Diab. Endocrinol. 8, 462–464 (2020).
Bommarito, P. A. et al. Prenatal Exposure to Nonpersistent Chemicals and Fetal-to-Childhood Growth Trajectories. Epidemiology 35, 874–884 (2024).
Wager, J. L. & Thompson, J. A. Development and Child Health in a World of Synthetic Chemicals. Pediatr. Res. 97, 1833–1839 (2025).
Edaes, F. S. & de Souza, C. B. Bps and Bpf Are as Carcinogenic as Bpa and Are Not Viable Alternatives for Its Replacement. Endocr. Metab. Immune Disord. Drug Targets 22, 927–934 (2022).
Martín-Carrasco, I., Carbonero-Aguilar, P., Dahiri, B., Moreno, I. M. & Hinojosa, M. Comparison between Pollutants Found in Breast Milk and Infant Formula in the Last Decade: A Review. Sci. Total Environ. 875, 162461 (2023).
Rivard, C. et al. Detection of Titanium Dioxide Particles in Human, Animal and Infant Formula Milk. Sci. Total Environ. 994, 180040 (2025).
Gatti, A. M. et al. Heavy Metal Nanoparticle Detection in Human and Formula Milk. Foods 13, 3178 (2024).
Serreau, R., Terbeche, Y. & Rigourd, V. Pollutants in Breast Milk: A Scoping Review of the Most Recent Data in 2024. Healthcare (Basel) 12, 680 (2024).
Tian, Y. et al. Occurrence and Nationwide Risk Assessment of Typical Food Processing Contaminants in Human Milk in China. J. Agric Food Chem. 73, 6917–6930 (2025).
Zhang, H. et al. Infantile Internal and External Exposure to Neonicotinoid Insecticides: A Comparison of Levels across Various Sources. Environ. Sci. Technol. 57, 5358–5367 (2023).
Lorber, M. & Toms, L. L. Use of a Simple Pharmacokinetic Model to Study the Impact of Breast-Feeding on Infant and Toddler Body Burdens of Pcb 153, Bde 47, and Dde. Chemosphere 185, 1081–1089 (2017).
Witczak, A., Pohoryło, A. & Abdel-Gawad, H. Endocrine-Disrupting Organochlorine Pesticides in Human Breast Milk: Changes During Lactation. Nutrients 13, 229 (2021).
Mekonen, S., Ambelu, A., Wondafrash, M., Kolsteren, P. & Spanoghe, P. Exposure of Infants to Organochlorine Pesticides from Breast Milk Consumption in Southwestern Ethiopia. Sci. Rep. 11, 22053 (2021).
Kao, C. C. et al. Residue Levels of Organochlorine Pesticides in Breast Milk and Its Associations with Cord Blood Thyroid Hormones and the Offspring’s Neurodevelopment. Int. J. Environ. Res. Public Health 16, 1438 (2019).
Barceló, D., Picó, Y. & Alfarhan, A. H. Microplastics: Detection in Human Samples, Cell Line Studies, and Health Impacts. Environ. Toxicol. Pharm. 101, 104204 (2023).
Landrigan, P. J. et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 89, 23 (2023).
Di Renzo, G. C. et al. International Federation of Gynecology and Obstetrics Opinion on Reproductive Health Impacts of Exposure to Toxic Environmental Chemicals. Int J. Gynaecol. Obstet. 131, 219–225 (2015).
LaKind, J. S., Naiman, J., Verner, M. A., Lévêque, L. & Fenton, S. Per- and Polyfluoroalkyl Substances (PFAS) in Breast Milk and Infant Formula: A Global Issue. Environ. Res. 219, 115042 (2023).
Dickerson, A. S., Frndak, S., DeSantiago, M., Mohan, A. & Smith, G. S. Environmental Exposure Disparities and Neurodevelopmental Risk: A Review. Curr. Environ. Health Rep. 10, 73–83 (2023).
Cathey, A. L. et al. Exploratory Profiles of Phenols, Parabens, and Per- and Poly-Fluoroalkyl Substances among Nhanes Study Participants in Association with Previous Cancer Diagnoses. J. Expo. Sci. Environ. Epidemiol. 33, 687–698 (2023).
Nair, A. A. et al. Environmental Exposure Disparities in Ultrafine Particles and Pm(2.5) by Urbanicity and Socio-Demographics in New York State, 2013-2020. Environ. Res. 239, 117246 (2023).
Sargis, R. M., Heindel, J. J. & Padmanabhan, V. Interventions to Address Environmental Metabolism-Disrupting Chemicals: Changing the Narrative to Empower Action to Restore Metabolic Health. Front Endocrinol. 10, 33 (2019).
Reams, M. A. & Irving, J. K. Applying Community Resilience Theory to Engagement with Residents Facing Cumulative Environmental Exposure Risks: Lessons from Louisiana’s Industrial Corridor. Rev. Environ. Health 34, 235–244 (2019).
Meek, J. Y. & Noble, L. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 150 (2022).
Kim, D. H. Transfusion Practice in Neonates. Korean J. Pediatr. 61, 265–270 (2018).
Navarro, M., Negre, S., Golombek, S., Matoses, M. L. & Vento, M. Intravenous Immune Globulin: Clinical Applications in the Newborn. NeoReviews 11, e370–e378 (2010).
Haass, K. A., Sapiano, M. R. P., Savinkina, A., Kuehnert, M. J. & Basavaraju, S. V. Transfusion-Transmitted Infections Reported to the National Healthcare Safety Network Hemovigilance Module. Transfus. Med. Rev. 33, 84–91 (2019).
Scott, S. R. & Wu, Z. Risks and Challenges of Hiv Infection Transmitted Via Blood Transfusion. Biosaf. Health 01, 124–128 (2019).
Dodd, R. Y. et al. Screening Blood Donors for Hiv, Hcv, and Hbv at the American Red Cross: 10-Year Trends in Prevalence, Incidence, and Residual Risk, 2007 to 2016. Transfus. Med Rev. 34, 81–93 (2020).
CDC. Clinical Testing Guidance for Blood Safety, https://www.cdc.gov/blood-safety/hcp/diagnosis-testing/index.html (2025).
Sherief, L. M. et al. Cmv, B and C Hepatitis among Multi-Transfused Hereditary Hemolytic Anemia Children: An Updated Egyptian Experience. Ital. J. Pediatr. 47, 117 (2021).
Bianchi, M. et al. Infectious Complications in Neonatal Transfusion: Narrative Review and Personal Contribution. Transfus. Apher. Sci. 59, 102951 (2020).
Delaney, M. et al. Postnatal Cytomegalovirus Infection: A Pilot Comparative Effectiveness Study of Transfusion Safety Using Leukoreduced-Only Transfusion Strategy. Transfusion 56, 1945–1950 (2016).
Chaudhary, N., Kilpatrick, R. & Singh, R. Red Cell Transfusion Related Toxic Metals Exposure for Fetus and Newborns: An under-Recognized Public Health Concern. Pediatr. Res. 97, 473–474 (2025).
Chen, M. et al. Environmental Lead Risk in the 21st Century. Commun. Earth Environ. 6, 776 (2025).
Puia-Dumitrescu, M. et al. Patterns of Phlebotomy Blood Loss and Transfusions in Extremely Low Birth Weight Infants. J. Perinatol. 39, 1670–1675 (2019).
Raffaeli, G. et al. Iron Homeostasis Disruption and Oxidative Stress in Preterm Newborns. Nutrients 12 (2020).
Ng, P. C. et al. Hepatic Iron Storage in Very Low Birthweight Infants after Multiple Blood Transfusions. Arch. Dis. Child Fetal Neonatal Ed. 84, F101–F105 (2001).
Park, S. H. & Kim, H. M. The Iron Status of Very Low Birth Weight Infants Receiving Multiple Erythrocyte Transfusions During Hospitalization in the Neonatal Intensive Care Unit. Pediatr. Gastroenterol. Hepatol. Nutr. 18, 100–107 (2015).
Perrone, S., Tataranno, L. M., Stazzoni, G., Ramenghi, L. & Buonocore, G. Brain Susceptibility to Oxidative Stress in the Perinatal Period. J. Matern. Fetal Neonatal Med. 28, 2291–2295 (2015).
Wang-Rodriguez, J. et al. Immune Response to Blood Transfusion Invery-Low-Birthweight Infants. Transfusion 40, 25–34 (2000).
Youssef, L. A. & Spitalnik, S. L. Transfusion-Related Immunomodulation: A Reappraisal. Curr. Opin. Hematol. 24, 551–557 (2017).
Muszynski, J. A. et al. Transfusion-Related Immunomodulation: Review of the Literature and Implications for Pediatric Critical Illness. Transfusion 57, 195–206 (2017).
Yang, Y. et al. The Effect of Immunoglobulin Treatment for Hemolysis on the Incidence of Necrotizing Enterocolitis - a Meta-Analysis. Eur. Rev. Med. Pharm. Sci. 20, 3902–3910 (2016).
Valieva, O. A., Strandjord, T. P., Mayock, D. E. & Juul, S. E. Effects of Transfusions in Extremely Low Birth Weight Infants: A Retrospective Study. J. Pediatr. 155, 331–337.e331 (2009).
Zhang, Z., Huang, X. & Lu, H. Association between Red Blood Cell Transfusion and Bronchopulmonary Dysplasia in Preterm Infants. Sci. Rep. 4, 4340 (2014).
Korhonen, P., Tammela, O., Koivisto, A. M., Laippala, P. & Ikonen, S. Frequency and Risk Factors in Bronchopulmonary Dysplasia in a Cohort of Very Low Birth Weight Infants. Early Hum. Dev. 54, 245–258 (1999).
Mally, P. et al. Association of Necrotizing Enterocolitis with Elective Packed Red Blood Cell Transfusions in Stable, Growing, Premature Neonates. Am. J. Perinatol. 23, 451–458 (2006).
Josephson, C. D. et al. Do Red Cell Transfusions Increase the Risk of Necrotizing Enterocolitis in Premature Infants? J. Pediatr. 157, 972–978.e971-973 (2010).
Blau, J. et al. Transfusion-Related Acute Gut Injury: Necrotizing Enterocolitis in Very Low Birth Weight Neonates after Packed Red Blood Cell Transfusion. J. Pediatr. 158, 403–409 (2011).
Marin, T. et al. Red Blood Cell Transfusion-Related Necrotizing Enterocolitis in Very-Low-Birthweight Infants: A near-Infrared Spectroscopy Investigation. Transfusion 53, 2650–2658 (2013).
El-Dib, M., Narang, S., Lee, E., Massaro, A. N. & Aly, H. Red Blood Cell Transfusion, Feeding and Necrotizing Enterocolitis in Preterm Infants. J. Perinatol. 31, 183–187 (2011).
Christensen, R. D. et al. Association, among Very-Low-Birthweight Neonates, between Red Blood Cell Transfusions in the Week after Birth and Severe Intraventricular Hemorrhage. Transfusion 54, 104–108 (2014).
Christensen, R. D. Associations between “Early” Red Blood Cell Transfusion and Severe Intraventricular Hemorrhage, and between “Late” Red Blood Cell Transfusion and Necrotizing Enterocolitis. Semin. Perinatol. 36, 283–289 (2012).
Baer, V. L., Lambert, D. K., Henry, E., Snow, G. L. & Christensen, R. D. Red Blood Cell Transfusion of Preterm Neonates with a Grade 1 Intraventricular Hemorrhage Is Associated with Extension to a Grade 3 or 4 Hemorrhage. Transfusion 51, 1933–1939 (2011).
Inder, T. E., Clemett, R. S., Austin, N. C., Graham, P. & Darlow, B. A. High Iron Status in Very Low Birth Weight Infants Is Associated with an Increased Risk of Retinopathy of Prematurity. J. Pediatr. 131, 541–544 (1997).
Dani, C. et al. The Role of Blood Transfusions and Iron Intake on Retinopathy of Prematurity. Early Hum. Dev. 62, 57–63 (2001).
Bernard, L. et al. Association between urinary metabolites and the exposure of intensive care newborns to plasticizers of medical devices used for their care management. Metabolites 11 (2021).
Panneel, L. et al. Ongoing Exposure to Endocrine Disrupting Phthalates and Alternative Plasticizers in Neonatal Intensive Care Unit Patients. Environ. Int. 186, 108605 (2024).
Macleod, D. J. et al. Androgen Action in the Masculinization Programming Window and Development of Male Reproductive Organs. Int J. Androl. 33, 279–287 (2010).
Bernard, L. et al. Medical Devices Used in NICU: The Main Source of Plasticisers’ Exposure of Newborns. Sci. Total Environ. 858, 159994 (2023).
Iribarne-Durán, L. M. et al. Presence of Bisphenol a and Parabens in a Neonatal Intensive Care Unit: An Exploratory Study of Potential Sources of Exposure. Environ. Health Perspect. 127, 117004 (2019).
Panneel, L., Malarvannan, G., Jorens, P. G., Covaci, A. & Mulder, A. Plasticizers in the Neonatal Intensive Care Unit: A Review on Exposure Sources and Health Hazards. Crit. Rev. Environ. Sci. Technol. 52, 3947–3972 (2022).
Keleş, E. et al. Phthalate Exposure Induces Cell Death and Ferroptosis in Neonatal Microglial Cells. Turk. J. Med Sci. 54, 1102–1115 (2024).
Panneel, L. et al. One Year Respiratory and Neurodevelopmental Outcome of Premature Neonates after Exposure to Plasticizers in the Neonatal Intensive Care Unit - a Prospective Cohort Study. Environ. Res. 274, 121266 (2025).
El-Metwally, D. et al. Urinary Metabolites of Volatile Organic Compounds of Infants in the Neonatal Intensive Care Unit. Pediatr. Res. 83, 1158–1164 (2018).
Almeida, M. O., Lanza, M. R. V. & Honorio, K. M. A Study of Possible Substitutes for the Endocrine Disruptor Dehp in Two Hormone Receptors. J. Biomol. Struct. Dyn. 40, 12516–12525 (2022).
Funding
Supported by Internal Funds, Department of Pediatrics, Tufts University School of Medicine, Boston, MA.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the conceptualization and drafting of the final review article. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kilpatrick, R., Chaudhary, N., Eze-Njoku, C. et al. Environmental exposures in the neonatal intensive care unit impacting neurodevelopmental outcomes for neonates. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04743-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04743-1