Abstract
Background
Necrotizing enterocolitis (NEC) is a severe gastrointestinal disorder in preterm infants. The interplay between mitochondrial metabolism and immune inflammation in its development is not fully understood.
Methods
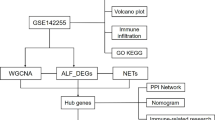
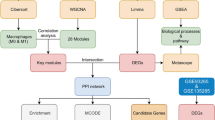
Single-cell data were analyzed using dimensionality reduction, clustering, and the high-dimensional weighted gene co-expression network analysis (hdWGCNA) algorithm to identify key gene modules in monocytes. GSE46619 was integrated with the MitoCarta3.0 to identify mitochondria-associated differentially expressed genes (MitoDEGs). Acyl-CoA synthetase long-chain family member 1 (ACSL1) was selected as a candidate. Immune infiltration was evaluated via the CIBERSORT algorithm, and a competing endogenous RNA (ceRNA) regulatory network was constructed using Cytoscape. The expression and function of ACSL1 were validated both in vivo and in vitro, using immunohistochemistry (IHC), qRT-PCR, western blot, and siRNA knockdown.
Results
A key monocyte subset was identified in NEC. Integrated analysis revealed three MitoDEGs (ACSL1, SOD2, SLC25A37) were linked to NEC, with ACSL1 showing the most significant upregulation. ACSL1 expression correlated strongly with immune cell infiltration and was confirmed to be elevated in vivo and in vitro models. Knocking down ACSL1 suppressed lipopolysaccharide (LPS)-induced inflammatory factor expression and ROS production.
Conclusion
ACSL1 plays a critical role in the pathogenesis of NEC, suggesting its potential as a novel biomarker.
Impact
-
Our study reveals massive monocyte infiltration and identifies the mitochondria-related gene ACSL1 as highly expressed and functionally significant in NEC.
-
This is the first integrated analysis (single-cell, hdWGCNA, MitoCarta3.0) pinpointing ACSL1 as a novel immunometabolic hub specific to NEC pathophysiology.
-
ACSL1 provides a crucial mechanistic link between mitochondrial function and NEC development.
-
It significantly correlates with key immune cells (neutrophils/mast cells/macrophages/T cells), highlighting its role in NEC immune dysregulation.
-
These findings reveal a critical role of ACSL1 in NEC pathogenesis, highlighting its potential as a novel biomarker.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated for this manuscript have been included in this article. The datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Niño, D. F., Sodhi, C. P. & Hackam, D. J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 13, 590–600 (2016).
Nolan, L. S., Wynn, J. L. & Good, M. Exploring clinically-relevant experimental models of neonatal shock and necrotizing enterocolitis. Shock 53, 596–604 (2020).
Jones, I. H. & Hall, N. J. Contemporary outcomes for infants with necrotizing enterocolitis-A systematic review. J. Pediatr. 220, 86–92.e83 (2020).
Egozi, A. et al. Single-cell atlas of the human neonatal small intestine affected by necrotizing enterocolitis. PLoS Biol. 21, e3002124 (2023).
Zhang, H., Feng, Y. W. & Yao, Y. M. Potential therapy strategy: Targeting mitochondrial dysfunction in sepsis. Mil. Med. Res. 5, 41 (2018).
Lee, H., Jeon, J. H. & Kim, E. S. Mitochondrial dysfunctions in T cells: Focus on inflammatory bowel disease. Front. Immunol. 14, 1219422 (2023).
Tiku, V., Tan, M. W. & Dikic, I. Mitochondrial functions in infection and immunity. Trends Cell Biol. 30, 263–275 (2020).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Liu, B. et al. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G573–G584 (2013).
Verma, A. et al. The role of the mitochondrial protein VDAC1 in inflammatory bowel disease: a potential therapeutic target. Mol. Ther. 30, 726–744 (2022).
Leek, J. T. et al. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 11, 733–739 (2010).
Dong, B. et al. Single-cell analysis supports a luminal-neuroendocrine transdifferentiation in human prostate cancer. Commun. Biol. 3, 778 (2020).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Li, R., Zhao, M., Miao, C., Shi, X. & Lu, J. Identification and validation of key biomarkers associated with macrophages in nonalcoholic fatty liver disease based on hdWGCNA and machine learning. Aging (Albany NY) 15, 15451–15472 (2023).
Morabito, S., Reese, F., Rahimzadeh, N., Miyoshi, E. & Swarup, V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 3, 100498 (2023).
Chan, K. Y. et al. Genome-wide expression profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in human intestinal tissues: dysregulation of functional pathways. Ann. Surg. 260, 1128–1137 (2014).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Li, J. H., Liu, S., Zhou, H., Qu, L. H. & Yang, J. H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42, D92–D97 (2014).
Furió-Tarí, P., Tarazona, S., Gabaldón, T., Enright, A. J. & Conesa, A. spongeScan: A web for detecting microRNA binding elements in lncRNA sequences. Nucleic Acids Res 44, W176–W180 (2016).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504 (2003).
Tian, Y. et al. A Neonatal BALB/c mouse model of necrotizing enterocolitis. J. Vis. Exp. https://doi.org/10.3791/63252 (2021).
Jilling, T., Lu, J., Jackson, M. & Caplan, M. S. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr. Res. 55, 622–629 (2004).
Sun, H. et al. Single-cell RNA-seq analysis identifies meniscus progenitors and reveals the progression of meniscus degeneration. Ann. Rheum. Dis. 79, 408–417 (2020).
Zhao, X., Wang, Q., Wang, W., Chen, X. & Lu, S. Study on molecular mechanism of intervertebral disc degeneration by single cell hdWGCNA combined with transcriptome sequencing. Noncoding RNA Res. 10, 177–191 (2025).
Gan, Y. et al. Spatially defined single-cell transcriptional profiling characterizes diverse chondrocyte subtypes and nucleus pulposus progenitors in human intervertebral discs. Bone Res. 9, 37 (2021).
Zhao, J., Yin, L. & He, L. The MicroRNA landscapes profiling reveals potential signatures of necrotizing enterocolitis in infants. J. Comput. Biol. 27, 30–39 (2020).
Rich, B. S. & Dolgin, S. E. Necrotizing enterocolitis. Pediatr. Rev. 38, 552–559 (2017).
Ho, G. T. & Theiss, A. L. Mitochondria and inflammatory bowel diseases: Toward a stratified therapeutic intervention. Annu. Rev. Physiol. 84, 435–459 (2022).
Haque, P. S., Kapur, N., Barrett, T. A. & Theiss, A. L. Mitochondrial function and gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 21, 537–555 (2024).
Zhu, F. et al. Blockage of NLRP3 inflammasome activation ameliorates acute inflammatory injury and long-term cognitive impairment induced by necrotizing enterocolitis in mice. J. Neuroinflamm. 18, 66 (2021).
Duess, J. W. et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes 15, 2221470 (2023).
Olaloye, O. O. et al. CD16+CD163+ monocytes traffic to sites of inflammation during necrotizing enterocolitis in premature infants. J. Exp. Med. 218, e20200344 (2021).
Liu, Z. Y. et al. ACSL1, CH25H, GPCPD1, and PLA2G12A as the potential lipid-related diagnostic biomarkers of acute myocardial infarction. Aging (Albany NY) 15, 1394–1411 (2023).
Wang, C. H., Surbhi, Goraya, S., Byun, J. & Pennathur, S. Fatty acids and inflammatory stimuli induce expression of long-chain acyl-CoA synthetase 1 to promote lipid remodeling in diabetic kidney disease. J. Biol. Chem. 300, 105502 (2024).
Nakakuki, M., Kawano, H., Notsu, T. & Imada, K. Eicosapentaenoic acid suppresses palmitate-induced cytokine production by modulating long-chain acyl-CoA synthetase 1 expression in human THP-1 macrophages. Atherosclerosis 227, 289–296 (2013).
Kalugotla, G. et al. Frontline Science: Acyl-CoA synthetase 1 exacerbates lipotoxic inflammasome activation in primary macrophages. J. Leukoc. Biol. 106, 803–814 (2019).
Soppert, J., Lehrke, M., Marx, N., Jankowski, J. & Noels, H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 159, 4–33 (2020).
Li, X. et al. Endothelial acyl-CoA synthetase 1 is not required for inflammatory and apoptotic effects of a saturated fatty acid-rich environment. Arterioscler Thromb. Vasc. Biol. 33, 232–240 (2013).
Kim, S. H. & Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-A mini-review. Nutrients 10, 1137 (2018).
Khaloian, S. et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut 69, 1939–1951 (2020).
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to the study. J.W. and Q.Y. contributed to the study conception and design. Y.C. and K.G. conducted the experiments, collected and analyzed the data. N.C. wrote the original draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Gao, K., Chen, N. et al. Identification of key mitochondria-related genes in necrotizing enterocolitis using single-cell hdWGCNA and experimental verification. Pediatr Res (2026). https://doi.org/10.1038/s41390-026-04779-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-026-04779-x