Abstract
Study design
Mapping Review.
Objective
The objective of this study was to map out and characterize the quantity and quality of all published spinal cord injury (SCI) randomized controlled trials (RCTs) with respect to number, sample size, and methodological quality between January 1970 and December 2016.
Setting
Not applicable.
Methods
A literature search of multiple research databases was conducted. Studies adhering to the following criteria were included: the research design was an RCT; written in English; participants were >18 years; and the sample was >50% SCI. Data were extracted pertaining to author(s), year of publication, country of origin, initial and final sample size, intervention, and control. Methodological quality was assessed using the Physiotherapy Evidence Database (PEDro) tool. Data was assessed overall and by each year of publication.
Results
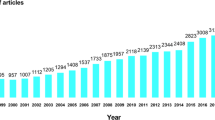
In total, 466 RCTs were published 1971–2016 on 29 primary topic areas, with Bladder (n = 78, 16.7%) most common, followed by Pain (n = 54, 11.6%), and Lower Limb (n = 45, 9.7%). Studies were published in 172 unique journals, with the most common being Spinal Cord (n = 68, 14.6%). The most common producer of studies was the United States (n = 191, 41.0%). RCT publications increased linearly until 2012 when the rate tripled, resulting in 40.8% published 2012–2016. A total of 247 (59.4%) RCTs had <30 subjects; there was no change in sample size over time (p = 0.770). The overall mean PEDro score was 5.56 (1.68); scores improved from 5.0 (1.4) in 1976 to 6.3 (1.9) in 2016 (F = 2.230, p < 0.001).
Conclusions
The number of SCI RCTs and their associated sample size remains low; however, methodological quality has improved over time.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings M. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;23:309–31.
Elliott TR. Registering randomized clinical trials and the case for CONSORT. Exp Cin Psychopharmacol. 2008;15:511–8.
Davidson KW, Goldstein M, Kaplan R, Kaufmann P, Knatterud G, Orleans T, et al. Evidence-based medicine: what is it and how do we achieve it? Ann Behav Med. 2003;26:161–71.
McIntyre A, Janzen S, Richardson M, Kwok C, Teasell R. An overview of acquired brain injury rehabilitation randomized controlled trials. Head Trauma Rehabil. 2015;30:E47–53.
McIntyre A, Richardson M, Janzen S, Hussein N, Teasell R. The evolution of stroke rehabilitation randomized controlled trials. Int J Stroke. 2014;9:789–92.
DePasse J,Park S,Eltorai A,Daniels A, Factors predicting publication of spinal cord injury trials registered on www.ClinicalTrials.gov. J Back Musculoskelet Rehabil. 2017;31:45–8.
Nowrouzi B, Assan-Lebbe A, Sharma B, Casole J, Nowrouzi-Kia B. Spinal cord injury: a review of the most-cited publications. Eur Spine J. 2017;26:28–39.
Kehn M, Kroll T. reporting trends of spinal cord injury research representation: a media content analysis. Disabil Health J. 2011;4:121–8.
Furlan J, Fehlings M. A Web-based systematic review on traumatic spinal cord injury comparing the “citation classics” with the consumers’ perspectives. J Neurotrauma. 2006;23:156–69.
Falagas ME, Grigori T, Ioannidou E. A systematic review of trends in the methodological quality of randomized controlled trials in various research fields. J Clin Epidemiol. 2009;62:227–31.
Sherrington C, Herbert R, Maher C, Moseley A. PEDro: a database of randomized trials and systematic reviews in physiotherapy. Man Ther. 2000;5:223–6.
Maher C, Sherrington C, Herbert R, Moseley A, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21.
De Morton N. The PEDro scale is a valid measure of the methodological quality of 7 clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33.
Foley N, Teasell R, Bhogal S, Speechley M. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10:1–7.
Sezer N, Akkus S, Ugurli F. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24–33.
Burke DC, Woodward JM. Pain and phantom sensations in spinal paralysis. In: Vinken PJ, Bruyn GW, (Eds). Handbook of Clinical Neurology. Amsterdam: North Holland Publishing Co.; 1976. p. 489–99.
Lammertse D, Tuszynski M, Steeves J, Curt A, Fawcett JW, Rask C. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord. 2007;45:232–42.
Andrews K. The limitations of randomized controlled trials in rehabilitation research. Clin Rehabil. 1991;5:5–8.
Turner L, Shamseer L, Altman D, Schulz K, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane Rev Syst Rev. 2002;1:60.
Pinto R, Elkins M, Moseley A, Sherrington C, Herbert R, Maher C, et al. Many randomized trials of physical therapy interventions are not adequately registered: a survey of 200 published trials. Phys Ther. 2003;93:299–309.
To M, Jones J, Emara M, Jadad A. Are reports of randomized controlled trials improving over time? A systematic review of 284 articles published in high-impact general and specialized medical journals. PLoS ONE. 2013;8:e84779.
Clark L, Schmidt U, Tharmanathan P, Adamson J, Hewitt C, Torgerson D. Allocation concealment: a methodological review. J Eval Clin Pract. 2013;19:708–12.
Foley N, Zettler L, Salter K, Bhogal S, Teasell R, Speechley M. In a review of stroke rehabilitation studies, concealed allocation was under reported. J Clin Epidemiol. 2009;62:766–70.
Hill C, LaValley M, Felson D. Discrepancy between published report and actual conduct of randomized clinical trials. J Clin Epidemiol. 2002;55:783–6.
Pildal J, Chan A, Hrobjartsson A, Forfang E, Altman D, Gotzsche P. Comparison of descriptions of allocation concealment in trial protocols and the published reports: Cohort study. BMJ. 2005;330:1049.
Montori V, Bhandari M, Devereaux P, Manns B, Ghali W, Guyatt G. In the dark: The reporting of blinding status in randomized controlled trials. J Clin Epidemiol. 2002;55:787–90.
Villamar M, Contreras V, Kuntz R, Fregni F. The reporting of blinding in physical medicine and rehabilitation randomized controlled trials: a systematic review. J Rehabil Med. 2013;45:6–13.
Devereaux P, Choi P, El-Dika S, Bhandari M, Montori V, Schunemann H, et al. An observational study found that authors of randomized controlled trials frequently use concealment of randomization and blinding, despite the failure to report these methods. J Clin Epidemiol. 2004;57:1232–6.
Soares H, Daniels S, Kumar A, Clarke M, Scott C, Swann S, et al. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ. 2004;328:22–4.
Mhaskar R, Djulbegovic B, Magazin A, Soares H, Kumar A. Published methodological quality of randomized controlled trials does not reflect the actual quality assessed in protocols. J Clin Epidemiol. 2012;65:602–9.
Schulz K, Grimes D. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696–700.
Hart T, Bagiella E. Design and implementation of clinical trials in rehabilitation research. Arch Phys Med Rehabil. 2012;93(8 Suppl):S117–26.
Boutron I, Guittet L, Estellat C, Moher D, Hrobjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007;4:e61.
Altman D, Doré C. Randomisation and baseline comparisons in clinical trials. Lancet. 1990;335:149–53.
Armijo-Olivo S, da Costa B, Cummings G, Ha C, Fuentes J, Saltaji H. PEDro or Cochrane to assess the quality of clinical trials? A meta-epidemiological study. PLoS ONE. 2015;10:e0132634.
Schulz K, Chalmers I, Grimes D, Altman D. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA. 1994;272:125–8.
Koletsi D, Pandis N, Polychronopoulou A, Eliades T. What’s in a title? An assessment of whether randomized controlled trial in a title means that it is one. Am J Orthod Dentofac Orthop. 2012;141:679–85.
Bland J, Altman D. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials. 2011;12:264.
Moseley A, Herbert R, Maher C, Sherrington C, Elkins M. PEDro scale can only rate what papers report. Aust J Physiother. 2008;54:288.
Viera A, Bangdiwala S. Eliminating bias in randomized controlled trials: importance of allocation concealment and masking. Fam Med. 2007;39:132–7.
Armijo-Olivo S, Fuentes J, Ospina M, Saltaji H, Hartling L. Inconsistency in the items included in tools used in general health research and physical therapy to evaluate the methodological quality of randomized controlled trials: a descriptive analysis. BMC Med Res Methodol. 2013;1:116.
Hernandez A, Steyerberg E, Taylor G, Marmarou A, Habbema J, Maas A. Subgroup analysis and covariate adjustment in randomized clinical trials of traumatic brain injury: a systematic review. Neurosurg. 2005;57:1244–53.
Page M, Higgins J, Clayton G, Sterne J, Hrobjartsson A, Savovic J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLoS ONE. 2016;11:e0159267.
Wood L, Egger M, Gluud L, Schulz K, Jüni P, Altman D, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336:601–5.
Author contributions
AMc was responsible for designing the review protocol, writing the protocol, conducting the search, screening for eligible studies, data extraction and analysis, drafting the findings, editing, and completing referencing and formatting. BB was responsible for assisting with conducting the search and screening for eligible studies, data extraction and analysis, writing the manuscript and completing referencing and formatting. SJ was responsible for assisting with conducting the search and screening for eligible studies, data extraction and analysis, writing the manuscript and completing referencing and formatting. JI and JW were responsible for writing the manuscript and completing referencing and formatting. JE and RT provided feedback on the manuscript.
Funding
We would like to gratefully acknowledge the Ontario Neurotrauma Foundation and the Rick Hansen Foundation for their generous support.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
McIntyre, A., Benton, B., Janzen, S. et al. A mapping review of randomized controlled trials in the spinal cord injury research literature. Spinal Cord 56, 725–732 (2018). https://doi.org/10.1038/s41393-018-0155-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-018-0155-2
This article is cited by
-
Developing spinal cord injury physiotherapy clinical practice guidelines: a qualitative study to determine how physiotherapists and people living with spinal cord injury use evidence
Spinal Cord (2023)
-
Work and SCI: a pilot randomized controlled study of an online resource for job-seekers with spinal cord dysfunction
Spinal Cord (2019)