Abstract
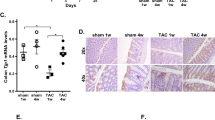
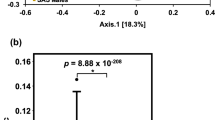
Mastitis is one of the most prevalent diseases in dairy farming worldwide. The gut microbiota plays an important role in the regulation of systemic and local inflammatory diseases, such as mastitis. However, the regulatory mechanism of the gut microbiota on mastitis is still unclear. Thus, the aim of this study was to investigate the function and regulatory mechanisms of the gut microbiota in host defense against mastitis caused by Staphylococcus aureus (S. aureus) infection. Increased blood-milk barrier permeability, and S. aureus-induced mastitis severity were observed gut microbiota-dysbiosis mice compared with those in control mice. Moreover, feces microbiota transplantation (FMT) to microbbiota-dysbiosis mice reversed these effects. Furthermore, established disruption of commensal homeostasis results in significantly increased abundance of pathogenic Enterobacter bacteria, while the relative abundance of short-chain fatty acid (SCFAs)-producing bacterial phyla (Firmicutes and Bacteroidetes) was significantly reduced. However, FMT to gut microbiota-dysbiosis mice reversed these changes. In addition, dysbiosis reduced the levels of SCFAs, and administration of sodium propionate, sodium butyrate, and probiotics (butyrate-producing bacteria) reversed the changes in the blood-milk barrier and reduced the severity of mastitis induced by S. aureus. In conclusion, this new finding demonstrated that the gut microbiota acts as a protective factor in host defense against mastitis and that targeting the gut-mammary gland axis represents a promising therapeutic approach for mastitis treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Melchior MB, Vaarkamp H, Fink-Gremmels J. Biofilms: a role in recurrent mastitis infections? Vet J. 2006;171:398–407.
Castilho IG, Dantas STA, Langoni H, Araujo JP Jr, Fernandes A,Jr, Alvarenga FCL, et al. Host-pathogen interactions in bovine mammary epithelial cells and HeLa cells by Staphylococcus aureus isolated from subclinical bovine mastitis. J Dairy Sci. 2017;100:6414–21.
Azara E, Longheu C, Sanna G, Tola S. Biofilm formation and virulence factor analysis of Staphylococcus aureus isolates collected from ovine mastitis. J Appl Microbiol. 2017;123:372–9.
Yue Y, Hymoller L, Jensen SK, Lauridsen C, Purup S. Effects of vitamin D and its metabolites on cell viability and Staphylococcus aureus invasion into bovine mammary epithelial cells. Vet Microbiol. 2017;203:245–51.
Smith GW, Lyman RL, Anderson KL. Efficacy of vaccination and antimicrobial treatment to eliminate chronic intramammary Staphylococcus aureus infections in dairy cattle. J Am Vet Med Assoc. 2006;228:422–5.
Ziesch M, Wente N, Zhang Y, Zaremba W, Engl S, Kromker V. Noninferiority trial investigating the efficacy of a nonantibiotic intramammary therapy in the treatment of mild-to-moderate clinical mastitis in dairy cows with longer lasting udder diseases. J Vet Pharm Ther. 2018;41:11–21.
Coyte KZ, Rakoff-Nahoum S. Understanding competition and cooperation within the mammalian gut microbiome. Curr Biol. 2019;29:R538–R544.
Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, et al. Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. J Lipid Res. 2017;58:1399–416.
Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24:660–72.
Vetizou M, Daillere R, Zitvogel L. Gut microbiota and efficacy of cancer therapies. Biol Aujourdhui. 2017;211:51–67.
Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J Nutr. 2017;147:1468S–1475S.
Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–39.
Zhao G, Vatanen T, Droit L, Park A, Kostic AD, Poon TW, et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc Natl Acad Sci USA. 2017;114:E6166–E6175.
Ma C, Zhao J, Xi X, Ding J, Wang H, Zhang H, et al. Bovine mastitis may be associated with the deprivation of gut Lactobacillus. Benef Microbes. 2016;7:95–102.
Addis MF, Tanca A, Uzzau S, Oikonomou G, Bicalho RC, Moroni P. The bovine milk microbiota: insights and perspectives from -omics studies. Mol Biosyst. 2016;12:2359–72.
Ma C, Sun Z, Zeng BH, Huang S, Zhao J, Zhang Y, et al. Cow-to-mouse fecal transplantations suggest intestinal microbiome as one cause of mastitis. Microbiome. 2018;6:200.
Guidry AJ, O’Brien CN, Douglass LW. A bovine mammary endothelial/epithelial cell culture model of the blood/milk barrier. Can J Vet Res. 1998;62:117–21.
Wall SK, Hernandez-Castellano LE, Ahmadpour A, Bruckmaier RM, Wellnitz O. Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. J Dairy Sci. 2016;99:7544–53.
Al-Asmakh M, Stukenborg JB, Reda A, Anuar F, Strand ML, Hedin L, et al. The gut microbiota and developmental programming of the testis in mice. PLoS One. 2014;9:e103809.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158.
Schuijt TJ, Lankelma JM, Scicluna BP, e Melo FD, Roelofs JJTH, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–83.
Tian ZH, Liu J, Liao MY, Li WJ, Zou JQ, Han XX, et al. Beneficial effects of fecal microbiota transplantation on ulcerative colitis in mice. Dig Dis Sci. 2016;61:2262–71.
Fu YH, Zhou ES, Wei ZK, Liang DJ, Wang W, Wang TC, et al. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Febs J. 2014;281:2543–57.
Cani PD, Amar J, Iglesias MA, Knauf C, Neynhck A, Alessi MC, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Ann Nutr Metab. 2007;51:79–79.
Kobayashi K, Oyama S, Numata A, Rahman MM, Kumura H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. Plos One. 2013;8:e62187.
Nguyen DA, Parlow AF, Neville MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol. 2001;170:347–56.
Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–R91.
Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol. 2007;12:25–35.
Mehrzad J, Duchateau L, Burvenich C. Viability of milk neutrophils and severity of bovine coliform mastitis. J Dairy Sci. 2004;87:4150–62.
Wellnitz O, Wall SK, Saudenova M, Bruckmaier RM. Effect of intramammary administration of prednisolone on the blood-milk barrier during the immune response of the mammary gland to lipopolysaccharide. Am J Vet Res. 2014;75:595–601.
Wellnitz O, Zbinden C, Huang X, Bruckmaier RM. Short communication: Differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J Dairy Sci. 2016;99:4851–6.
Harrington M. For lack of gut microbes, the blood-brain barrier ‘leaks’. Lab Anim (NY). 2015;44:6.
Michel L, Prat A. One more role for the gut: microbiota and blood brain barrier. Ann Transl Med. 2016;4:15.
Rosean CB, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 2019;79:3662–75.
Yuan L, Zhang SR, Li H, Yang F, Mushtaq N, Ullah S, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–93.
Chou DW, Wu SL, Lee CT. Intensive care unit-acquired complicated necrotizing pneumonia caused by Enterobacter cloacae: a case report. Intractable Rare Dis. 2018;7:283–6.
Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY). 2016;8:1049–63.
Gao J, Barkema HW, Zhang L, Liu G, Deng Z, Cai L, et al. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J Dairy Sci. 2017;100:4797–806.
Gutierrez-Chavez AJ, Martinez-Ortega EA, Valencia-Posadas M, Leon-Galvan MF, Fuente-Salcido N, Bideshi DK, et al. Potential use of Bacillus thuringiensis bacteriocins to control antibiotic-resistant bacteria associated with mastitis in dairy goats. Folia Microbiol. 2016;61:11–9.
Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119.
Ciarlo E, Heinonen T, Herderschee J, Fenwick C, Mombelli M, Le Roy D, et al. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep. 2016;6:37944.
Wang J, Wei Z, Zhang X, Wang Y, Yang Z, Fu Y. Propionate protects against lipopolysaccharide-induced mastitis in mice by restoring blood-milk barrier disruption and suppressing inflammatory response. Front Immunol. 2017;8:1108.
Lasitschka F, Giese T, Paparella M, Kurzhals SR, Wabnitz G, Jacob K, et al. Human monocytes downregulate innate response receptors following exposure to the microbial metabolite n-butyrate. Immun Inflamm Dis. 2017;5:480–92.
Pant K, Saraya A, Venugopal SK. Oxidative stress plays a key role in butyrate-mediated autophagy via Akt/mTOR pathway in hepatoma cells. Chem-Biol Interact. 2017;273:99–106.
Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64.
Liu Q, Liu J, Roschmann KI, van Egmond D, Golebski K, Fokkens WJ, et al. Histone deacetylase inhibitors up-regulate LL-37 expression independent of toll-like receptor mediated signalling in airway epithelial cells. J Inflamm. 2013;10:15.
Kanai T, Mikami Y, Hayashi A. A breakthrough in probiotics: clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J Gastroenterol. 2015;50:928–39.
Zhang J, Chen XY, Liu P, Zhao JB, Sun J, Guan WY, et al. Dietary clostridium butyricum induces a phased shift in fecal microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model. J Agr Food Chem. 2018;66:5157–66.
Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJF, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015;26:1877–88.
Tholozan JL, Samain E, Grivet JP, Moletta R, Dubourguier HC, Albagnac G. Reductive carboxylation of propionate to butyrate in methanogenic ecosystems. Appl Environ Microbiol. 1988;54:441–5.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (Nos. 31772812, 31972749, 31472248, 30972225, 30771596) and China Postdoctoral Science Foundation funded project (2016M600233).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The permit number (20170317) was assigned by the Institutional Animal Care and Use Committee (IACUC) of Jilin University for animal experiments approvals. The full proposal was reviewed by the IACUC ethics committee, which approved the animal care and use permit license. All experiments comply with the manual of the care and use of laboratory animals published by the US National Institutes of Health.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, X., Guo, J., Zhao, C. et al. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J 14, 1897–1910 (2020). https://doi.org/10.1038/s41396-020-0651-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-020-0651-1
This article is cited by
-
Production, Mechanisms, and Therapeutic Strategies of Tryptophan Metabolites in CNS Diseases
Molecular Neurobiology (2026)
-
Butyrate enhances gut dysbiosis by activating the cAMP/PKA/CREB signaling pathway to inhibit the progression of endometrial carcinoma
BMC Microbiology (2025)
-
An investigation into variations in the raw milk microbiota of dairy cows in Ningxia, China: effects of season, farm, parity, and health status
BMC Veterinary Research (2025)
-
Investigating the Anna Karenina principle of the breast microbiome
BMC Microbiology (2025)
-
Vagus nerve stimulation alleviates S. aureus-induced mastitis by regulating gut microbiota S24-7-PPARγ and NF-ΚB/NLRP3 signaling in mice
Journal of Neuroinflammation (2025)