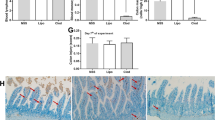
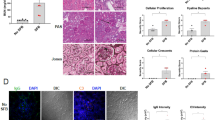
Abstract
Leptospirosis is a re-emerging zoonotic disease worldwide. Intestinal bleeding is a common but neglected symptom in severe leptospirosis. The regulatory mechanism of the gut microbiota on leptospirosis is still unclear. In this study, we found that Leptospira interrogans infection changed the composition of the gut microbiota in mice. Weight loss and an increased leptospiral load in organs were observed in the gut microbiota-depleted mice compared with those in the control mice. Moreover, fecal microbiota transplantation (FMT) to the microbiota-depleted mice reversed these effects. The phagocytosis response and inflammatory response in bone marrow-derived macrophages and thioglycolate-induced peritoneal macrophages were diminished in the microbiota-depleted mice after infection. However, the phagocytosis response and inflammatory response in resident peritoneal macrophage were not affected in the microbiota-depleted mice after infection. The diminished macrophage disappearance reaction (bacterial entry into the peritoneum acutely induced macrophage adherence to form local clots and out of the fluid phase) led to an increased leptospiral load in the peritoneal cavity in the microbiota-depleted mice. In addition, the impaired capacity of macrophages to clear leptospires increased leptospiral dissemination in Leptospira-infected microbiota-depleted mice. Our study identified the microbiota as an endogenous defense against L. interrogans infection. Modulating the structure and function of the gut microbiota may provide new individualized preventative strategies for the control of leptospirosis and related spirochetal infections.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–96.
Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898.
Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat Rev Microbiol. 2017;15:297–307.
Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97.
Jimenez JIS, Marroquin JLH, Richards GA, Amin P. Leptospirosis: report from the task force on tropical diseases by the world federation of societies of intensive and critical care medicine. J Crit Care. 2018;43:361–5.
Guerrier G, D’Ortenzio E. The Jarisch-Herxheimer reaction in leptospirosis: a systematic review. PLoS One. 2013;8:e59266.
Alventosa Mateu C, Plana Campos L, Larrey Ruíz L, Acedo Mayordomo R, Sanchís Artero L, Peño Muñoz L, et al. [Gastrointestinal bleeding and acute hepatic failure by leptospirosis: an entity that should not be forgotten]. Rev Gastroenterol Peru. 2017;37:96–9.
Miyahara S, Saito M, Kanemaru T, Villanueva SY, Gloriani NG, Yoshida S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int J Exp Pathol. 2014;95:271–81.
Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–90.
Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–83.
Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–81.
Lankelma JM, Birnie E, Weehuizen TAF, Scicluna BP, Belzer C, Houtkooper RH, et al. The gut microbiota as a modulator of innate immunity during melioidosis. PLoS Negl Trop Dis. 2017;11:e0005548.
Nair N, Guedes MS, Werts C, Gomes-Solecki M. The route of infection with Leptospira interrogans serovar Copenhageni affects the kinetics of bacterial dissemination and kidney colonization. PLoS Negl Trop Dis. 2020;14:e0007950.
Zhang N, Czepielewski RS, Jarjour NN, Erlich EC, Esaulova E, Saunders BT, et al. Expression of factor V by resident macrophages boosts host defense in the peritoneal cavity. J Exp Med. 2019;216:1291–300.
Gomes CK, Guedes M, Potula HH, Dellagostin OA, Gomes-Solecki M. Sex matters: male hamsters are more susceptible to lethal infection with lower doses of pathogenic Leptospira than female hamsters. Infect Immun. 2018;86:e00369-18.
Vemuri R, Sylvia KE, Klein SL, Forster SC, Plebanski M, Eri R, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity, and disease susceptibility. Semin Immunopathol. 2019;41:265–75.
Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–20.
Walker WE, Bozzi AT, Goldstein DR. IRF3 contributes to sepsis pathogenesis in the mouse cecal ligation and puncture model. J Leukoc Biol. 2012;92:1261–8.
Spalinger MR, Schwarzfischer M, Hering L, Shawki A, Sayoc A, Santos A, et al. Loss of PTPN22 abrogates the beneficial effect of cohousing-mediated fecal microbiota transfer in murine colitis. Mucosal Immunol. 2019;12:1336–47.
Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–82.
Lacroix-Lamande S, d’Andon MF, Michel E, Ratet G, Philpott DJ, Girardin SE, et al. Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome. J Immunol. 2012;188:2805–14.
Toma C, Okura N, Takayama C, Suzuki T. Characteristic features of intracellular pathogenic Leptospira in infected murine macrophages. Cell Microbiol. 2011;13:1783–92.
Zhang W, Xie X, Wang J, Song N, Lv T, Wu D, et al. Increased inflammation with crude E. coli LPS protects against acute leptospirosis in hamsters. Emerg Microbes Infect. 2020;9:140–7.
Rojas P, Monahan AM, Schuller S, Miller IS, Markey BK, Nally JE. Detection and quantification of leptospires in urine of dogs: a maintenance host for the zoonotic disease leptospirosis. Eur J Clin Microbiol Infect Dis. 2010;29:1305–9.
Cao Y, Xie X, Zhang W, Wu D, Tu C. Low-dose norfloxacin-treated leptospires induce less IL-1beta release in J774A.1cells following discrepant leptospiral gene expression. Micro Pathog. 2018;119:125–30.
Zhang W, Zhang N, Xie X, Guo J, Jin X, Xue F, et al. Toll-like receptor 2 agonist Pam3CSK4 alleviates the pathology of leptospirosis in hamster. Infect Immun. 2016;84:3350–7.
Scott NA, Andrusaite A, Andersen P, Lawson M, Alcon-Giner C, Leclaire C, et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med. 2018;10:eaao4755.
Lu X, Liu J, Zhang N, Fu Y, Zhang Z, Li Y, et al. Ripened pu-erh tea extract protects mice from obesity by modulating gut microbiota composition. J Agric Food Chem. 2019;67:6978–94.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809.
Chen X, Li SJ, Ojcius DM, Sun AH, Hu WL, Lin X, et al. Mononuclear-macrophages but not neutrophils act as major infiltrating anti-leptospiral phagocytes during leptospirosis. PLoS One. 2017;12:e0181014.
Ferrer MF, Scharrig E, Charo N, Ripodas AL, Drut R, Carrera Silva EA, et al. Macrophages and galectin 3 control bacterial burden in acute and subacute murine leptospirosis that determines chronic kidney fibrosis. Front Cell Infect Microbiol. 2018;8:384.
Santecchia I, Vernel-Pauillac F, Rasid O, Quintin J, Gomes-Solecki M, Boneca IG, et al. Innate immune memory through TLR2 and NOD2 contributes to the control of Leptospira interrogans infection. PLoS Pathog. 2019;15:e1007811.
Maruoka T, Nikaido Y, Miyahara S, Katafuchi E, Inamasu Y, Ogawa M, et al. Correlation between renal distribution of leptospires during the acute phase and chronic renal dysfunction in a hamster model of infection with Leptospira interrogans. PLoS Negl Trop Dis. 2021;15:e0009410.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52.
Burrello C, Garavaglia F, Cribiu FM, Ercoli G, Lopez G, Troisi J, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun. 2018;9:5184.
Zhang Q, Hu J, Feng JW, Hu XT, Wang T, Gong WX, et al. Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol. 2020;21:99.
Alavi S, Mitchell JD, Cho JY, Liu R, Macbeth JC, Hsiao A. Interpersonal gut microbiome variation drives susceptibility and resistance to Cholera infection. Cell. 2020;181:1533–46 e13.
Winglee K, Eloe-Fadrosh E, Gupta S, Guo H, Fraser C, Bishai W. Aerosol Mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLoS One. 2014;9:e97048.
Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, et al. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe. 2018;24:296–307 e7.
Becattini S, Sorbara MT, Kim SG, Littmann EL, Dong Q, Walsh G, et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe. 2021;29:378–93 e5.
Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell. 2021;184:615–27 e17.
Li XV, Leonardi I, Iliev ID. Gut mycobiota in immunity and inflammatory disease. Immunity. 2019;50:1365–79.
Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–95.
Sencio V, Barthelemy A, Tavares LP, Machado MG, Soulard D, Cuinat C, et al. Gut dysbiosis during Influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30:2934–47 e6.
Potula HH, Richer L, Werts C, Gomes-Solecki M. Pre-treatment with Lactobacillus plantarum prevents severe pathogenesis in mice infected with Leptospira interrogans and may be associated with recruitment of myeloid cells. PLoS Negl Trop Dis. 2017;11:e0005870.
Coutinho ML, Matsunaga J, Wang LC, de la Pena Moctezuma A, Lewis MS, Babbitt JT, et al. Kinetics of Leptospira interrogans infection in hamsters after intradermal and subcutaneous challenge. PLoS Negl Trop Dis. 2014;8:e3307.
Prochazkova P, Roubalova R, Dvorak J, Kreisinger J, Hill M, Tlaskalova-Hogenova H, et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes. 2021;13:1–25.
Zilber AL, Belli P, Grezel D, Artois M, Kodjo A, Djelouadji Z. Comparison of mucosal, subcutaneous and intraperitoneal routes of rat Leptospira infection. PLoS Negl Trop Dis. 2016;10:e0004569.
Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–89.
Mishra A, Lai GC, Yao LJ, Aung TT, Shental N, Rotter-Maskowitz A, et al. Microbial exposure during early human development primes fetal immune cells. Cell. 2021;184:3394–3409.e20.
Mandal RK, Denny JE, Namazzi R, Opoka RO, Datta D, John CC, et al. Dynamic modulation of spleen germinal center reactions by gut bacteria during Plasmodium infection. Cell Rep. 2021;35:109094.
Wang C, Li Q, Ren J. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Front Immunol. 2019;10:1873.
Brugiroux S, Beutler M, Pfann C, Garzetti D, Ruscheweyh HJ, Ring D, et al. Genome-guided design of a defined mouse microbiota that confers colonization resistance against Salmonella enterica serovar Typhimurium. Nat Microbiol. 2016;2:16215.
Acknowledgements
We thank Dr. Sukanya Narasimhan (Yale School of Medicine, New Haven, USA) for carefully editing the manuscript and Dr. Xiaokui Guo (Shanghai Jiao Tong University, Shanghai, China) for providing Leptospira interrogans serovar Lai strain Lai (56601).
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 32172872 and 31572582), the Fundamental Research Funds for the Central Universities, China Postdoctoral Science Foundation funded project (2020M670860) and Interdisciplinary Research Funding Program for Doctoral Postgraduates of Jilin University (101832020DJX096).
Author information
Authors and Affiliations
Contributions
XX and JL share co-first authorship. JL and YC conceived ideas, and XX and WZ designed experiments. XX, JL, RT, XW and XC performed the mouse experiments. JL, SZ and JC conducted the bacteriology experiments. XX and JL analyzed data. WZ and YC performed bioinformatics analysis. XX and JL wrote the original draft. WZ and YC supervised the study and reviewed the final version of the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xie, X., Liu, J., Chen, X. et al. Gut microbiota involved in leptospiral infections. ISME J 16, 764–773 (2022). https://doi.org/10.1038/s41396-021-01122-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-021-01122-6