Abstract
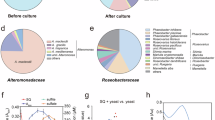
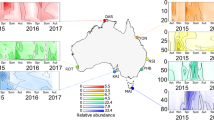
Sulfoquinovose (SQ) is one of the most abundant organosulfur compounds in the biosphere, and its biosynthesis and degradation can represent an important contribution to the sulfur cycle. To data, in marine environments, the microorganisms capable of metabolising SQ have remained unidentified and the sources of SQ are still uncertain. Herein, the marine Roseobacter clade bacteria (RCB) Dinoroseobacter shibae DFL 12 and Roseobacter denitrificans OCh 114 were found to grow using SQ as the sole source of carbon and energy. In the presence of SQ, we identified a set of highly up-regulated proteins encoded by gene clusters in these two organisms, of which four homologues to proteins in the SQ monooxygenase pathway of Agrobacterium fabrum C58 may confer the ability to metabolise SQ to these marine bacteria. The sulfite released from SQ desulfonation by FMN-dependent SQ monooxygenase (SmoC) may provide bacteria with reduced sulfur for assimilation, while proteins associated with sulfite production via assimilatory sulfate reduction were significantly down-regulated. Such SQ catabolic genes are restricted to a limited number of phylogenetically diverse bacterial taxa with the predominate genera belonging to the Roseobacter clade (Roseobacteraceae). Moreover, transcript analysis of Tara Oceans project and coastal Bohai Sea samples provided additional evidence for SQ metabolism by RCB. SQ was found to be widely distributed in marine phytoplankton and cyanobacteria with variable intracellular concentrations ranging from micromolar to millimolar levels, and the amounts of SQ on particulate organic matter in field samples were, on average, lower than that of dimethylsulfoniopropionate (DMSP) by one order of magnitude. Together, the phototroph-derived SQ actively metabolised by RCB represents a previously unidentified link in the marine sulfur cycle.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Proteomic data have been uploaded to the National Omics Data Encyclopedia (NODE, https://www.biosino.org/node/) database with the accession number OEP003145.
References
Snow AJD, Burchill L, Sharma M, Davies GJ, Williams SJ. Sulfoglycolysis: Catabolic pathways for metabolism of sulfoquinovose. Chem Soc Rev. 2021;50:13628–45.
Van Mooy BAS, Rocap G, Fredricks HF, Evans CT, Devol AH. Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc Natl Acad Sci USA 2006;103:8607–12.
Wu J, Sunda W, Boyle EA, Karl DM. Phosphate depletion in the western North Atlantic. Ocean Sci 2000;289:759–62.
Goddard-Borger ED, Williams SJ. Sulfoquinovose in the biosphere: occurrence, metabolism and functions. Biochem J. 2017;474:827–49.
Harwood JL, Nicholls RG. The plant sulpholipid- a major component of the sulphur cycle. Biochem Soc Trans. 1979;7:440–7.
Moran MA, Durham BP. Sulfur metabolites in the pelagic ocean. Nat Rev Microbiol. 2019;17:665–78.
Tang K. Chemical diversity and biochemical transformation of biogenic organic sulfur in the ocean. Front Mar Sci. 2020;7:68.
Denger K, Weiss M, Felux AK, Schneider A, Mayer C, Spiteller D, et al. Sulphoglycolysis in Escherichia coli K-12 closes a gap in the biogeochemical sulphur cycle. Nature 2014;507:114–7.
Hanson BT, Kits KD, Loffler J, Burrichter AG, Fiedler A, Denger K, et al. Sulfoquinovose is a select nutrient of prominent bacteria and a source of hydrogen sulfide in the human gut. ISME J. 2021;15:2779–91.
Strickland TC, Fitzgerald JW. Mineralization of sulfur in sulfoquinovose by forest soils. Soil Biol Biochem. 1983;15:347–9.
Felux AK, Spiteller D, Klebensberger J, Schleheck D. Entner-Doudoroff pathway for sulfoquinovose degradation in Pseudomonas putida SQ1. Proc Natl Acad Sci USA 2015;112:E4298–E305.
Frommeyer B, Fiedler AW, Oehler SR, Hanson BT, Loy A, Franchini P, et al. Environmental and intestinal phylum Firmicutes bacteria metabolize the plant sugar sulfoquinovose via a 6-deoxy-6-sulfofructose transaldolase pathway. Iscience. 2020;23:101510.
Roy AB, Hewlins MJE, Ellis AJ, Harwood JL, White GF. Glycolytic breakdown of sulfoquinovose in bacteria: A missing link in the sulfur cycle. Appl Environ Microbiol. 2003;69:6434–41.
Liu J, Wei Y, Ma K, An J, Liu X, Liu Y, et al. Mechanistically diverse pathways for sulfoquinovose degradation in bacteria. ACS Catal. 2021;11:14740–50.
Zhang S, Li Z, Yan Y, Zhang C, Li J, Zhao B. Bacillus urumqiensis sp. nov., a moderately haloalkaliphilic bacterium isolated from a salt lake. Int J Syst Evol Microbiol. 2016;66:2305–12.
Durham BP, Sharma S, Luo H, Smith CB, Amin SA, Bender SJ, et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc Natl Acad Sci USA 2015;112:453–7.
Chen X, Liu L, Gao X, Dai X, Han Y, Chen Q, et al. Metabolism of chiral sulfonate compound 2,3-dihydroxypropane-1-sulfo-nate (DHPS) by Roseobacter bacteria in marine environment. Environ Int. 2021;157:106829.
Liu J, Wei Y, Lin L, Teng L, Yin J, Lu Q, et al. Two Radical-dependent mechanisms for anaerobic degradation of the globally abundant Organosulfur Compound Dihydroxypropanesulfonate. Proc Natl Acad Sci USA 2020;117:15599.
Xing M, Wei Y, Zhou Y, Zhang J, Lin L, Hu Y, et al. Radical-mediated C-S bond cleavage in C2 sulfonate degradation by anaerobic bacteria. Nat Commun. 2019;10:1609.
Sharma M, Lingford JP, Petricevic M, Snow AJD, Zhang Y, Jarva MA, et al. Oxidative desulfurization pathway for complete catabolism of sulfoquinovose by bacteria. Proc Natl Acad Sci USA 2022;119:e2116022119.
Scholz SS, Serif M, Schleheck D, Sayer MDJ, Cook AM, Kupper FC. Sulfoquinovose metabolism in marine algae. Bot Mar. 2021;64:301–12.
Abayakoon P, Epa R, Petricevic M, Bengt C, Mui JWY, van der Peet PL, et al. Comprehensive synthesis of substrates, intermediates, and products of the sulfoglycolytic Embden-Meyerhoff-Parnas pathway. J Org Chem. 2019;84:2901–10.
Denger K, Smits THM, Cook AM. L-Cysteate sulpho-lyase, a widespread pyridoxal 5 ‘-phosphate-coupled desulphonative enzyme purified from Silicibacter pomeroyi DSS-3. Biochem J. 2006;394:657–64.
Guillard RRL. Culture of Phytoplankton for Feeding Marine Invertebrates. Smith WL, Chanley MH, (eds): Springer US; 1975. Boston, MA. pp 29–60.
Moore LR, Coe A, Zinser ER, Saito MA, Sullivan MB, Lindell D, et al. Culturing the marine cyanobacterium Prochlorococcus. Limnol Oceanogr Methods. 2007;5:353–62.
Waterbury J, Watson S, Valois F, Franks D. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Platt T, Li WKW, (eds). Department of Fisheries and Oceans, Ottawa 1986. pp 71–120.
Olenina I, Hajdu S, Edler L, Andersson A, Wasmund N, Busch S, et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Balt Sea Environ Proc. 2006;106:144.
Zheng Q, Wang Y, Lu J, Lin W, Chen F, Jiao N. Metagenomic and metaproteomic insights into photoautotrophic and heterotrophic interactions in a Synechococcus culture. mbio 2020;11:e03261–19.
Partensky F, Hess WR, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63:106–27.
Han Y, Zhang M, Chen X, Zhai W, Tan E, Tang K. Transcriptomic evidences for microbial carbon and nitrogen cycles in the deoxygenated seawaters of Bohai Sea. Environ Int. 2022;158:106889.
Li WKW. Primary production of prochlorophytes, cyanobacteria, and eukaryotic ultraphytoplankton - measurements from flow cytometric sorting. Limnol Oceanogr. 1994;39:169–75.
Denger K, Ruff A, Rein U, Cook AM. Sulphoacetaldehyde sulpho-lyase (EC 4.4.1.12) from Desulfonispora thiosulfatigenes: purification, properties and primary sequence. Biochem J. 2001;357:581–6.
Ismail R, Lee HY, Mahyudin NA, Abu, Bakar F. Linearity study on detection and quantification limits for the determination of avermectins using linear regression. J Food Drug Anal. 2014;22:407–12.
Klemetsen T, Raknes IA, Fu J, Agafonov A, Balasundaram SV, Tartari G, et al. The MAR databases: development and implementation of databases specific for marine metagenomics. Nucleic Acids Res. 2018;46:D692–D9.
Suzek BE, Huang H, McGarvey P, Mazumder R, Wu CH. UniRef: comprehensive and non-redundant UniProt reference clusters. Bioinformatics 2007;23:1282–8.
Rozewicki J, Li S, Amada KM, Standley DM, Katoh K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 2019;47:W5–W10.
Schuller DJ, Reisch CR, Moran MA, Whitman WB, Lanzilotta WN. Structures of dimethylsulfoniopropionate-dependent demethylase from the marine organism Pelagabacter ubique. Protein Sci. 2012;21:289–98.
Bharath SR, Bisht S, Harijan RK, Savithri HS, Murthy MR. Structural and mutational studies on substrate specificity and catalysis of Salmonella typhimurium D-cysteine desulfhydrase. PLoS One. 2012;7:e36267.
Chartron J, Carroll KS, Shiau C, Gao H, Leary JA, Bertozzi CR, et al. Substrate Recognition, Protein Dynamics, and Iron-Sulfur Cluster in Pseudomonas aeruginosa Adenosine 5′-Phosphosulfate Reductase. J Mol Biol. 2006;364:152–69.
Davis KM, Altmyer M, Martinie RJ, Schaperdoth I, Krebs C, Bollinger JM Jr, et al. Structure of a Ferryl Mimic in the Archetypal Iron(II)- and 2-(Oxo)-glutarate-Dependent Dioxygenase, TauD. Biochemistry 2019;58:4218–23.
Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: Making protein folding accessible to all. Nat Methods. 2022;19:679–82.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021;596:583–9.
Zhang C, Shine M, Pyle AM, Zhang Y. US-align: universal structure alignments of proteins, nucleic acids, and macromolecular complexes. Nat Methods. 2022;19:1109–15.
Xu J, Zhang Y. How significant is a protein structure similarity with TM-score = 0.5? Bioinformatics 2010;26:889–95.
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2014;32:268–74.
Villar E, Vannier T, Vernette C, Lescot M, Cuenca M, Alexandre A, et al. The Ocean Gene Atlas: exploring the biogeography of plankton genes online. Nucleic Acids Res. 2018;46:W289–W95.
Vernette C, Henry N, Lecubin J, de Vargas C, Hingamp P, Lescot M. The Ocean barcode atlas: A web service to explore the biodiversity and biogeography of marine organisms. Mol Ecol Resour. 2021;21:1347–58.
Paoli L, Ruscheweyh H-J, Forneris CC, Hubrich F, Kautsar S, Bhushan A, et al. Biosynthetic potential of the global ocean microbiome. Nature 2022;607:111–8.
Sunagawa S, Acinas SG, Bork P, Bowler C, Acinas SG, Babin M, et al. Tara Oceans: towards global ocean ecosystems biology. Nat Rev Microbiol. 2020;18:428–45.
Acinas SG, Sánchez P, Salazar G, Cornejo-Castillo FM, Sebastián M, Logares R, et al. Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities. Commun Biol. 2021;4:604.
Biller SJ, Berube PM, Dooley K, Williams M, Satinsky BM, Hackl T, et al. Marine microbial metagenomes sampled across space and time. Sci Data. 2018;5:180176.
Pachiadaki MG, Brown JM, Brown J, Bezuidt O, Berube PM, Biller SJ, et al. Charting the Complexity of the Marine Microbiome through Single-Cell Genomics. Cell 2019;179:1623–35.
Delmont TO, Quince C, Shaiber A, Esen ÖC, Lee STM, Rappé MS, et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol. 2018;3:804–13.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9.
Subramanian B, Gao S, Lercher MJ, Hu S, Chen W-H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47:W270–W5.
Xing M, Wei Y, Zhou Y, Zhang J, Lin L, Hu Y, et al. Radical-mediated C-S bond cleavage in C2 sulfonate degradation by anaerobic bacteria. Nat Commun. 2019;10:1609.
Biebl H, Allgaier M, Tindall BJ, Koblizek M, Lunsdorf H, Pukall R, et al. Dinoroseobacter shibae gen. nov., sp nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int J Syst Evol Microbiol. 2005;55:1089–96.
Fu H, Uchimiya M, Gore J, Moran MA. Ecological drivers of bacterial community assembly in synthetic phycospheres. Proc Natl Acad Sci USA 2020;117:3656–62.
Chen I-MA, Chu K, Palaniappan K, Ratner A, Huang J, Huntemann M, et al. The IMG/M data management and analysis system v.7: content updates and new features. Nucleic Acids Res. 2022. https://doi.org/10.1093/nar/gkac976.
Shiba T. Roseobacter litoralis gen. nov., sp. nov., and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst Appl Microbiol. 1991;14:140–5.
Kopriva S, Calderwood A, Weckopp SC, Koprivova A. Plant sulfur and big data. Plant Sci. 2015;241:1–10.
Simon J, Kroneck PMH. Microbial sulfite respiration. Adv Micro Physiol. 2013;62:45–117.
Gonzalez JM, Covert JS, Whitman WB, Henriksen JR, Mayer F, Scharf B, et al. Silicibacter pomeroyi sp nov and Roseovarius nubinhibens sp nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. Int J Syst Evol Microbiol. 2003;53:1261–9.
Liang KYH, Orata FD, Boucher YF, Case RJ. Roseobacters in a sea of poly- and paraphyly: whole genome-based taxonomy of the family Rhodobacteraceae and the proposal for the split of the “Roseobacter clade” into a novel family, Roseobacteraceae fam. nov. Front Microbiol. 2021;12:683109.
Howard EC, Sun S, Biers EJ, Moran MA. Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environ Microbiol. 2008;10:2397–410.
Howard EC, Henriksen JR, Buchan A, Reisch CR, Buergmann H, Welsh R, et al. Bacterial taxa that limit sulfur flux from the ocean. Science. 2006;314:649–52.
Durham BP, Boysen AK, Carlson LT, Groussman RD, Heal KR, Cain KR, et al. Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat Microbiol. 2019;4:1706–15.
Smetacek V. Diatoms and the ocean carbon cycle. Protist 1999;150:25–32.
Stoecker DK, Lavrentyev PJ. Mixotrophic plankton in the polar seas: A pan-Arctic review. Front Mar Sci. 2018;5:292.
Turner SM, Malin G, Liss PS, Harbour DS, Holligan PM. The seasonal-variation of dimethyl sulfide and dimethylsulfoniopropionate concentrations in nearshore waters. Limnol Oceanogr. 1988;33:364–75.
Belviso S, Kim S-K, Rassoulzadegan F, Krajka B, Nguyen BC, Mihalopoulos N, et al. Production of dimethylsulfonium propionate (DMSP) and dimethylsulfide (DMS) by a microbial food web. Limnol Oceanogr. 1990;35:1810–21.
Simo R, Pedros-Alio C, Malin G, Grimalt JO. Biological turnover of DMS, DMSP and DMSO in contrasting open-sea waters. Mar Ecol Prog Ser. 2000;203:1–11.
Flombaum P, Gallegos JL, Gordillo RA, Rincon J, Zabala LL, Jiao N, et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA 2013;110:9824–9.
Gasparovic B, Penezic A, Frka S, Kazazic S, Lampitt RS, Holguin FO, et al. Particulate sulfur-containing lipids: Production and cycling from the epipelagic to the abyssopelagic zone. Deep Sea Res Part I Oceanogr Res Pap. 2018;134:12–22.
Zhan P, Tang K, Chen X, Yu L. Complete genome sequence of Maribacter sp T28, a polysaccharide-degrading marine flavobacteria. J Biotechnol. 2017;259:1–5.
Van Mooy BAS, Fredricks HF. Bacterial and eukaryotic intact polar lipids in the eastern subtropical South Pacific: Water-column distribution, planktonic sources, and fatty acid composition. Geochim Cosmochim Acta. 2010;74:6499–516.
Popendorf KJ, Tanaka T, Pujo-Pay M, Lagaria A, Courties C, Conan P, et al. Gradients in intact polar diacylglycerolipids across the Mediterranean Sea are related to phosphate availability. Biogeosciences 2011;8:3733–45.
Acknowledgements
We would like to thank Prof Spencer J. Williams (University of Melbourne, Australia) for providing the SQ standard. We also thank Prof Kunshan Gao (Xiamen University, China) and Center for Collections of Marine Algae (Xiamen University, China) for providing algae strains.
Funding
This study was supported by the National Natural Science Foundation of China project (92251306), the National Key Research and Development Program of China (2020YFA0608300), and the NSFC project (42276120, 42076160, 42188102).
Author information
Authors and Affiliations
Contributions
KT conceived the study, analyzed data, and wrote the manuscript. LL performed the experiments, analyzed data, wrote and edited the manuscript. XC sampled, performed the experiments, analyzed data, and wrote the manuscript. JY performed the experiments. XM performed the experiments. YuH sampled, and analyzed data. YajieH performed the experiments. All authors have approved the submitted final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Chen, X., Ye, J. et al. Sulfoquinovose is a widespread organosulfur substrate for Roseobacter clade bacteria in the ocean. ISME J 17, 393–405 (2023). https://doi.org/10.1038/s41396-022-01353-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-022-01353-1
This article is cited by
-
A novel roseosiphovirus infecting dinoroseobacter shibae DFL12T represents a new genus
BMC Genomics (2025)
-
Cosmopolitan marine bacteria facilitate a vast phytoplankton-derived sulfonate-based carbon flow through sulfoquinovosidases
Nature Communications (2025)
-
Metagenomic analysis reveals genetic coupling between TonB-dependent transporters and extracellular enzymes in coastal bacterial communities
Marine Life Science & Technology (2025)
-
A Broad-Spectrum α-Glucosidase of Glycoside Hydrolase Family 13 from Marinovum sp., a Member of the Roseobacter Clade
Applied Biochemistry and Biotechnology (2024)