Abstract
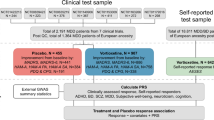
Late-life depression (LLD) is often accompanied by medical comorbidities such as psychiatric disorders and cardiovascular diseases, posing challenges to antidepressant treatment. Recent studies highlighted significant associations between treatment-resistant depression (TRD) and polygenic risk score (PRS) for attention deficit hyperactivity disorder (ADHD) in adults as well as a negative association between antidepressant symptom improvement with both schizophrenia and bipolar. Here, we sought to validate these findings with symptom remission in LLD. We analyzed the Incomplete Response in Late Life Depression: Getting to Remission (IRL-GRey) sample consisting of adults aged 60+ with major depression (N = 342) treated with venlafaxine for 12 weeks. We constructed PRSs for ADHD, depression, schizophrenia, bipolar disorder, neuroticism, general intelligence, antidepressant symptom remission and antidepressant percentage symptom improvement using summary statistics from the Psychiatric Genomics Consortium and the GWAS Catalog. Logistic regression was used to test the association of PRSs with venlafaxine symptom remission and percentage symptom improvement, co-varying for the genomic principal components, age, sex and depressive symptoms severity at baseline. We found a nominal (i.e., p value ≤ 0.05) association between symptom remission and both PRS for ADHD and (OR = 1.36 [1.07, 1.73], p = 0.011) and PRS for bipolar disorder (OR = 0.75 [0.58, 0.97], p = 0.031), as well as between percentage symptom improvement and PRS for general intelligence (beta = 6.81 (SE = 3.122), p = 0.03). However, the ADHD association was in the opposite direction as expected, and both associations did not survive multiple testing corrections. Altogether, these findings suggest that previous findings regarding ADHD PRS and antidepressant response (measured with various outcomes) do not replicate in older adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The GWAS summary statistics for the psychiatric disorders used in this analysis are publicly available from: the Psychiatric Genomics Consortium; https://www.med.unc.edu/pgc/download-results/, and the GWAS Catalogue; https://www.ebi.ac.uk/gwas.
Code availability
To obtain the results presented here, we mostly followed the tutorials and general usage available from PLINK 2.0 alpha; https://www.cog-genomics.org/plink/2.0/, PRSice-2; https://choishingwan.github.io/PRS-Tutorial/.
References
Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Institute for Health Metrics and Evaluation, Seattle, Washington: University of Washington; 2019. http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b.
The World Health Organization (WHO). Depression. 2022. https://www.who.int/news-room/fact-sheets/detail/depression.
Paolucci S. Epidemiology and treatment of post-stroke depression. Neuropsychiatr Dis Treat. 2008;4:145–54.
Alexopoulos GS. “Vascular Depression” hypothesis. Arch Gen Psychiatry. 1997;54:915. https://doi.org/10.1001/archpsyc.1997.01830220033006.
Davydow DS, Levine DA, Zivin K, Katon WJ, Langa KM. The association of depression, cognitive impairment without dementia, and dementia with risk of ischemic stroke: a cohort study. Psychosom Med. 2015;77:200–8.
Tiwari AK, Zai CC, Altar CA, Tanner JA, Davies PE, Traxler P, et al. Clinical utility of combinatorial pharmacogenomic testing in depression: a Canadian patient- and rater-blinded, randomized, controlled trial. Transl Psychiatry. 2022;12:101.
Murphy LE, Fonseka TM, Bousman CA, Müller DJ. Gene-drug pairings for antidepressants and antipsychotics: level of evidence and clinical application. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01340-6.
Marshe VS, Islam F, Maciukiewicz M, Bousman C, Eyre HA, Lavretsky H, et al. Pharmacogenetic implications for antidepressant pharmacotherapy in late-life depression: a systematic review of the literature for response, pharmacokinetics and adverse drug reactions. Am J Geriatr Psychiatry. 2020;28:609–29.
Meerman JJ, Ter Hark SE, Janzing JGE, Coenen MJH. The potential of polygenic risk scores to predict antidepressant treatment response in major depression: a systematic review. J Affect Disord. 2022;304:1–11.
Amare AT, Schubert KO, Tekola-Ayele F, Hsu YH, Sangkuhl K, Jenkins G, et al. Association of the polygenic scores for personality traits and response to selective serotonin reuptake inhibitors in patients with major depressive disorder. Front Psychiatry. 2018;9:65.
Amare AT, Schubert KO, Tekola-Ayele F, Hsu YH, Sangkuhl K, Jenkins G, et al. The association of obesity and coronary artery disease genes with response to SSRIs treatment in major depression. J Neural Transm. 2019;126:35–45.
Marshe VS, Maciukiewicz M, Hauschild AC, Islam F, Qin L, Tiwari AK, et al. Genome-wide analysis suggests the importance of vascular processes and neuroinflammation in late-life antidepressant response. Transl Psychiatry. 2021;11:127.
Pain O, Hodgson K, Trubetskoy V, Ripke S, Marshe VS, Adams MJ, et al. Identifying the common genetic basis of antidepressant response. Biol Psychiatry Glob Open Sci. 2022;2:115–26.
Fabbri C, Hagenaars SP, John C, Williams AT, Shrine N, Moles L, et al. Genetic and clinical characteristics of treatment-resistant depression using primary care records in two UK cohorts. Mol Psychiatry. 2021;26:3363–73.
Tansey KE, Guipponi M, Hu X, Domenici E, Lewis G, Malafosse A, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. 2013;73:679–82.
Biobank UK. UK Biobank Primary Care Linked Data. Version 1.0. 2019.
John C, Reeve NF, Free RC, Williams AT, Ntalla I, Farmaki AE, et al. Cohort profile: extended cohort for E-health, Environment and DNA (EXCEED). Int J Epidemiol. 2019;48:1734.
Incomplete Response in Late Life Depression: Getting to Remission (IRL GREY)—Full Text View—ClinicalTrials.Gov [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT00892047.
Lenze EJ, Mulsant BH, Blumberger DM, Karp JF, Newcomer JW, Anderson SJ, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–12.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022;604:502–8.
Jones L, Gordon-Smith K, Perry A. Genome-wide association study of over 40,000 bipolar disorder cases provides novel biological insights. Nat Genet. 2021. http://eprints.worc.ac.uk/10304/.
van den Berg SM, de Moor MHM, McGue M, Pettersson E, Terracciano A, Verweij KJH, et al. Harmonization of neuroticism and extraversion phenotypes across inventories and cohorts in the Genetics of Personality Consortium: an application of Item Response Theory. Behav Genet. 2014;44:295–313.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7.
Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529.
Lemieux Perreault LP, Legault MA, Asselin G, Dubé MP. genipe: an automated genome-wide imputation pipeline with automatic reporting and statistical tools. Bioinformatics. 2016;32:3661–3.
van Leeuwen EM, Kanterakis A, Deelen P, Kattenberg MV, Genome of the Netherlands Consortium, Slagboom PE, et al. Population-specific genotype imputations using minimac or IMPUTE2. Nat Protoc. 2015;10:1285–96.
Consortium GP, Auton A, Brooks LD, Durbin RM. A global reference for human genetic variation. Nature. 2015;526:68–74.
Loh PR, Danecek P, Palamara PF, Fuchsberger C. Reference-based phasing using the Haplotype Reference Consortium panel. Nature. 2016. https://idp.nature.com/authorize/casa?redirect_uri=https://www.nature.com/articles/ng.3679.pdf%3Forigin%3Dppub&casa_token=K0H1LVsq2I4AAAAA:5T4wwiAwm5ddV2nDaJVIMertO95YFBxYJv6-0WE-kmhZOAZWgDXQWo9d6J1NT1HqCmXqeP7Pbt78pCFPEXg.
Choi SW, O’Reilly PF. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience. 2019;8. https://doi.org/10.1093/gigascience/giz082.
Coombes BJ, Ploner A, Bergen SE, Biernacka JM. A principal component approach to improve association testing with polygenic risk scores. Genet Epidemiol. 2020;44:676–86.
Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–9.
Cinar O, Viechtbauer W. The poolr package for combining independent and dependent p values. J Stat Softw. 2022. http://statistik-jstat.uibk.ac.at/article/view/v101i01.
Li QS, Wajs E, Ochs-Ross R, Singh J, Drevets WC. Genome-wide association study and polygenic risk score analysis of esketamine treatment response. Sci Rep. 2020;10:12649.
Incomplete Response in Late Life Depression: Getting to Remission (IRL GREY)—Study Results—ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/results/NCT00892047?view=results.
Sirey JA, Woods A, Solomonov N, Evans L, Banerjee S, Zanotti P, et al. Treatment adequacy and adherence as predictors of depression response in primary care. Am J Geriatr Psychiatry. 2020;28:1164–71.
Hung CI. Factors predicting adherence to antidepressant treatment. Curr Opin Psychiatry. 2014;27:344–9.
Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–9.
Acknowledgements
This work was supported by funding from (1) CIHR #; (2) funding from CANSSI Ontario: Trainee SSME is a fellow of STAGE (Strategic Training for Advanced Genetic Epidemiology); (3) the CAMH Discovery Fund Postdoctoral Fellowship supporting SSME; (4) Temerty-Tanz-TDRA Research Fellowships supporting SSME. Computations were performed on the CAMH Specialized Computing Cluster (SCC). The SCC is funded by: The Canada Foundation for Innovation, Research Hospital Fund.
Author information
Authors and Affiliations
Contributions
The study was conceptualized by Samar S. M. Elsheikh (SSME) and Daniel J. Müller (DM). The methodology was designed by SSME, DM and Victoria S. Marshe (VM). Formal analysis of the data was performed by SSME. The manuscript was drafted by SSME. Comprehensive reviewing and editing of the manuscript were carried out by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elsheikh, S.S.M., Marshe, V.S., Men, X. et al. Polygenic score analyses on antidepressant response in late-life depression, results from the IRL-GRey study. Pharmacogenomics J 24, 38 (2024). https://doi.org/10.1038/s41397-024-00351-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41397-024-00351-0
This article is cited by
-
Genomic risk prediction for depression in a large prospective study of older adults of European descent
Molecular Psychiatry (2025)