Abstract
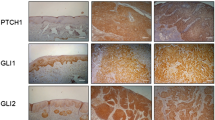
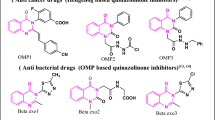
The G protein-coupled receptor (GPCR) smoothened (SMO) is a key signaling component of the sonic hedgehog (Hh) pathway and a clinically validated target for cancer treatment. The FDA-approved SMO inhibitors GDC-0449/Vismodegib and LDE225/Sonidegib demonstrated clinical antitumor efficacy. Nevertheless, relatively high percentage of treated patients would eventually develop acquired cross resistance to both drugs. Here, based on published structure and activity of GDC-0449 inhibitor class, we replaced its amide core with benzimidazole which retained bulk of the SMO-targeting activity as measured in our Hh/SMO/Gli1-reporter system. Synthesis and screening of multiple series of benzimidazole derivatives identified HH-1, HH-13, and HH-20 with potent target suppression (IC50: <0.1 μmol/L) in the reporter assays. In NIH3T3 cells stimulated with a secreted Hh (SHH), these inhibitors dose dependently reduced mRNA and protein expression of the endogenous pathway components PTCH-1, Gli1, and cyclin D1 resulting in growth inhibition via G0/G1 arrest. Mechanistically, the SMO-targeted growth inhibition involved downregulation of mTOR signaling inputs and readouts consistent with diminished mTORC1/mTORC2 functions and apoptosis. In mice, as with GDC-0449, orally administered HH inhibitors blocked paracrine activation of stromal Hh pathway in Calu-6 tumor microenvironment and attenuated growth of PTCH+/−/P53−/− medulloblastoma allograft tumors. Furthermore, HH-13 and HH-20 potently targeted the drug-resistant smoothened SMO-D473H (IC50: <0.2 μmol/L) compared to the poor inhibition by GDC-0449 (IC50: >60 μmol/L). These results identify HH-13 and HH-20 as potent inhibitors capable of targeting naïve and drug-resistant Hh/SMO-driven cancers. The current leads may be optimized to improve pharmaceutical property for potential development of new therapy for treatment of Hh pathway-driven cancers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ishibashi M, Mcmahon AP. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development. 2002;129:4807–19.
Pasca DMM, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11.
Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–22.
Yang L, Xie GQ, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–81.
Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal-cell carcinoma. Biochim Et Biophys Acta. 2010;1805:181–208.
Harris LG, Samant RS, Shevde LA. Hedgehog signaling: networking to nurture a pro-malignant tumor microenvironment. Mol Cancer Res. 2011;9:1165–74.
Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–61.
Athar M, Li C, Tang X, Chi S, Zhang X, Kim AL, et al. Inhibition ofsmoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation ofFas expression and apoptosis. Cancer Res. 2004;64:7545–52.
Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. The 12-month analysis from basal cell carcinoma outcomes with LDE225 treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016;75:113–25.
Von Hoff DD, Lorusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. New Engl J Med. 2009;361:1164–72.
Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7.
Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4.
Sharpe HJ, Pau G, Dijkgraaf GJ, Bassetseguin N, Modrusan Z, Januario T, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015;27:327–41.
Danial C, Sarin KY, Oro AE, Chang AL. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325–9.
Sharpe HJ, Pau G, Dijkgraaf GJ, et al. Genomic analysis ofsmoothened inhibitor resistance in basal cell carcinoma. J Cancer Cell. 2015;27:327–41.
Atwood SX, Sarin KY, Whitson RJ, Li JR, Kim G, Rezaee M, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. J Cancer Cell. 2015;27:342–53.
Ishii T, Shimizu Y, Nakashima K, Kondo S, Ogawa K, Sasaki S, et al. Inhibition mechanism exploration of investigational drug tak-441 as inhibitor against vismodegib-resistant smoothened mutant. Eur J Pharmacol. 2014;723:305–13.
Peukert S, He F, Dai M, Zhang R, Sun Y, Miller-Moslin K, et al. Cover picture: discovery of nvp-leq506, a second-generation inhibitor of smoothened (chemmedchem 8/2013). ChemMedChem. 2013;8:1261.
Lee MJ, Hatton BA, Villavicencio EH, Khanna PC, Friedman SD, Ditzler S, et al. Hedgehog pathway inhibitor saridegib (ipi-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci USA. 2012;109:7859–64.
Lang, H., Yu, K., & Zhao, H. Benzimidazole compound with antitumour activity as well as preparation method and application thereof. Patent Application CN201310139335.
Lang, H., Yu, K., & Zhao, H. Benzimidazole amide compound as well as preparation method and application thereof. Patent Application CN201310186206.
Koo J, Yue P, Deng X, Khuri FR, Sun SY. mTOR complex 2 stabilizes Mcl-1 protein by suppressing its glycogen synthase kinase 3-dependent and SCF-FBXW7-mediated degradation. Mol Cell Biol. 2015;35:2344–55.
Li X, Wang Z, Ma Q, Xu Q, Liu H, Duan W, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res. 2014;20:4326–38.
Lubik AA, Nouri M, Truong S, Ghaffari M, Adomat HH, Corey E, et al. Paracrine sonic hedgehog signaling contributes significantly to acquired steroidogenesis in the prostate tumor microenvironment. Int J Cancer. 2017;140:358–69.
Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, et al. GDC-0449—a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–81.
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international metaanalysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–84.
Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6:229–40.
Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis K, Combemale P, et al. LBA33 randomized, double-blind study of sonidegib (LDE225) in patients (PTS) with advanced basal cell carcinoma (BCC). Ann Oncol. 2014;25:1–41.
Chang ALS, Solomon JA, Hainsworth JD, Goldberg L, McKenna E, Day B-M, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60–9.
Sasaki K, Gotlib JR, Mesa RA, Newberry KJ, Ravandi F, Cortes JE, et al. Phase II evaluation of IPI-926, an oral hedgehog inhibitor, in patients with myelofibrosis. Leuk Lymphoma. 2015;56:2092–7.
Wagner AJ, Messersmith WA, Shaik MN, Li S, Zheng X, McLachlan KR, Cesari R, Courtney R, Levin WJ, El-Khoueiry AB. Aphase IstudyofPF-04449913,an oralhedgehog inhibitor,in patients with advanced solid tumors. Clin Cancer Res. 2015;21:1044–51.
Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29:2717–23.
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10.
Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by sonic hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–205.
Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med Oncol. 2015;32:368.
Kern D, Regl G, Hofbauer SW, Altenhofer P, Achatz G, Dlugosz A, et al. Hedgehog/GLI and PI3K signaling in the initiation and maintenance of chronic lymphocytic leukemia. Oncogene. 2015;34:5341–51.
Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70.
Metcalfe C, Alicke B, Crow A, Lamoureux M, Dijkgraaf GJ, Peale F, et al. PTEN loss mitigates the response of medulloblastoma to hedgehog pathway inhibition. Cancer Res. 2013;73:7034–42.
Sharma N, Nanta R, Sharma J, Gunewardena S, Singh KP, Shankar S, et al. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget. 2015;6:32039–60.
Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–44.
Yu K, Toral-barza L, Shi C, Zhang WG, Lucas J, Shor B, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–40.
Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–6.
Acknowledgements
This work was supported by a startup grant from Fudan University (EZF301002) and National Natural Science Foundation of China (81273367). We thank Fudan University School of Pharmacy Animal Facility and Instrument Center for excellent technical support.
Author contributions
Conception and design: H.L., K.Y. Development of methodology: H.Z., Q.L., K.Y. Acquisition of data: H.Z., Q.L., X.Z. Analysis and interpretation of data: H.Z., Q.L., X.Z. Writing, review, and/or revision of the manuscript: Q.L., H.L., K.Y. Administrative, technical, or material support: H.L., K.Y. Study supervision: K.Y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Qr., Zhao, H., Zhang, Xs. et al. Novel-smoothened inhibitors for therapeutic targeting of naïve and drug-resistant hedgehog pathway-driven cancers. Acta Pharmacol Sin 40, 257–267 (2019). https://doi.org/10.1038/s41401-018-0019-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0019-5
Keywords
This article is cited by
-
Pharmacological modulation of stem cells signaling pathway for therapeutic applications
Stem Cell Research & Therapy (2025)
-
Identification of Meibomian gland stem cell populations and mechanisms of aging
Nature Communications (2025)
-
Spelling Out CICs: A Multi-Organ Examination of the Contributions of Cancer Initiating Cells’ Role in Tumor Progression
Stem Cell Reviews and Reports (2022)
-
Identification of a potent antagonist of smoothened in hedgehog signaling
Cell & Bioscience (2021)
-
Pluripotent Stem Cells: Cancer Study, Therapy, and Vaccination
Stem Cell Reviews and Reports (2021)