Abstract
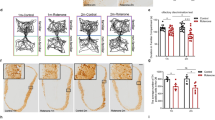
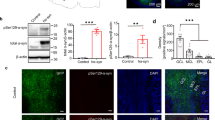
Olfactory bulb, as one of sensory organs opening to the outside, is susceptible to toxic environment and easy to deteriorate. Recent studies in Parkinson’s disease (PD) patients and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys have shown that abnormal α-synuclein is accumulated in the olfactory glomeruli, suggesting that the lesions of PD are not only confined to the substantia nigra (SN) but also located in the olfactory bulb. Thus, olfactory bulb might be the region of onset in PD pathogenesis and a targeted region for diagnosis and treatment of PD. However, the relationship between olfactory bulb and pathogenesis of PD remains unclear. In the present study, we investigated the inflammatory pathological alterations in olfactory bulb and the underlying mechanisms in chronic MPTP mice. Mice were treated with MPTP/P, i.e., MPTP (25 mg/kg, s.c.) plus probenecid (250 mg/kg, i.p.) every 4 days, for ten times. The mice displayed typical parkinsonian syndrome. Then we examined their olfactory function and the pathologic changes in olfactory bulb. The mice showed obvious olfactory dysfunction in a buried pellet test. Immunohistochemical studies revealed that tyrosine hydroxylase (TH) protein levels were significantly decreased, whereas abnormal α-synuclein was significantly increased in the olfactory bulbs. Furthermore, the olfactory bulbs in MPTP/P-treated mice showed significantly increased levels of interleukin-1β (IL-1β), caspase-1, glial fibrillary acidic protein (GFAP), Toll receptor 4 (TLR4), phosphorylation of p65, as well as activated molecules of NOD-like receptor protein 3 (NLRP3) that were associated with neuroinflammation. Our results demonstrate that MPTP/P-caused olfactory bulb damage might be related to NLRP3-mediated inflammation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Trist BG, Davies KM, Cottam V, Genoud S, Ortega R, Roudeau S, et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol. 2017;134:113–27.
Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52.
Mundinano IC, Hernandez M, Dicaudo C, Ordonez C, Marcilla I, Tunon MT, et al. Reduced cholinergic olfactory centrifugal inputs in patients with neurodegenerative disorders and MPTP-treated monkeys. Acta Neuropathol. 2013;126:411–25.
Rey NL, Wesson DW, Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis. 2018;109:226–48.
Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human alpha-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–73.
Hoglinger GU, Alvarez-Fischer D, Arias-Carrion O, Djufri M, Windolph A, Keber U, et al. A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015;130:333–48.
Kanaan NM, Kordower JH, Collier TJ. Age-related changes in glial cells of dopamine midbrain subregions in rhesus monkeys. Neurobiol Aging. 2010;31:937–52.
Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialog-Clin Neurosci. 2017;19:71–80.
Guo JD, Zhao X, Li Y, Li GR, Liu XL. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med. 2018;41:1817–25.
Niranjan R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem Int. 2018;120:13–20.
Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65.
Liu Y, Sun JD, Song LK, Li J, Chu SF, Yuan YH, et al. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav Brain Res. 2015;294:149–61.
Zhang QS, Heng Y, Chen Y, Luo P, Wen L, Zhang Z, et al. A novel bibenzyl compound (20C) protects mice from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid toxicity by regulating the alpha-synuclein-related inflammatory response. J Pharmacol Exp Ther. 2017;363:284–92.
Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96.
Clark AJ, Ritz B, Prescott E, Rod NH. Psychosocial risk factors, pre-motor symptoms and first-time hospitalization with Parkinson’s disease: a prospective cohort study. Eur J Neurol. 2013;20:1113–20.
Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Movement disorders: official journal of the Movement Disorder. Society. 2000;15:669–77.
Olanow CW, Schapira AH, Roth T. Waking up to sleep episodes in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder. Society. 2000;15:212–5.
Bohnen NI, Studenski SA, Constantine GM, Moore RY. Diagnostic performance of clinical motor and non-motor tests of Parkinson disease: a matched case-control study. Eur J Neurol. 2008;15:685–91.
Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:509.
Silveira-Moriyama L, Holton JL, Kingsbury A, Ayling H, Petrie A, Sterlacci W, et al. Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett. 2009;453:77–80.
Beach TG, White CL 3rd, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117:169–74.
Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80.
Barker, R. Environmental toxins and Parkinson’s disease. Trends Neurosci. 1989;12:182–3.
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–15.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40.
Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, et al. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314.
Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci: Off J Soc Neurosci. 2008;28:7687–98.
Melki R. Alpha-synuclein and the prion hypothesis in Parkinson's disease. Revue neurologique 2018;174:644-52.
Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–83.
Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893–901.
Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol. 2016;40:79–89.
Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–14.
Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83.
Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11:339–46.
Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, et al. NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212:1725–38.
Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76.
Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32.
Wen L, Zhang QS, Heng Y, Chen Y, Wang S, Yuan YH, et al. NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol Lett. 2018;288:1–8.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26.
Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–80.
Shao QH, Zhang XL, Yang PF, Yuan YH, Chen NH. Amyloidogenic proteins associated with neurodegenerative diseases activate the NLRP3 inflammasome. Int Immunopharmacol. 2017;49:155–60.
Hafner-Bratkovic I, Bencina M, Fitzgerald KA, Golenbock D, Jerala R. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1beta and neuronal toxicity. Cell Mol life Sci: CMLS. 2012;69:4215–28.
Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE. 2013;8:e55375.
Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder?. Mov Disord . 2013;28:31–40.
Litteljohn D, Mangano E, Clarke M, Bobyn J, Moloney K, Hayley S. Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s disease. Parkinson’s Dis. 2010;2011:713517.
Masters SL, O’Neill LA. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med. 2011;17:276–82.
Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci: Off J Soc Neurosci. 2005;25:6016–24.
Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–9.
Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97.
Shi F, Kouadir M, Yang Y. NALP3 inflammasome activation in protein misfolding diseases. Life Sci. 2015;135:9–14.
von Herrmann KM, Salas LA, Martinez EM, Young AL, Howard JM, Feldman MS, et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinson’s Dis. 2018;4:24.
Martinez EM, Young AL, Patankar YR, Berwin BL, Wang L, von Herrmann KM, et al. Editor’sHighlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting From Chronic Intragastric Rotenone Exposure in Mice. Toxicol Sci: Off J Soc Toxicol. 2017;159:64–75.
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73.
Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018; 10(465). pii: eaah4066. http://stm.sciencemag.org/content/10/465/eaah4066.full
Qiao C, Zhang Q, Jiang Q, Zhang T, Chen M, Fan Y, et al. Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease. J Neuroinflamm. 2018;15:193.
Omais S, Jaafar C, Ghanem N. “Till Death Do Us Part”: A potential irreversible link between aberrant cell cycle control and neurodegeneration in the adult olfactory bulb. Front Neurosci. 2018;12:144.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81573640, 81773925, and U1402221), the Beijing Natural Science Foundation (7161011), and Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University), Ministry of Education (P201701).
Author contributions
Y-hY and N-hC designed the research; YC, Q-sZ, Q-hS, SW, and H-bW performed the research; Q-hS, SW, and H-bW contributed analytic tools; YC and Q-sZ analyzed data; YC wrote the paper. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, Qs., Shao, Qh. et al. NLRP3 inflammasome pathway is involved in olfactory bulb pathological alteration induced by MPTP. Acta Pharmacol Sin 40, 991–998 (2019). https://doi.org/10.1038/s41401-018-0209-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0209-1
Keywords
This article is cited by
-
Loganic Acid Alleviates the Olfactory-Brain NLRP3 Inflammasome Activation and Rescues Dopaminergic Neurons in Experimental Models of Parkinson’s Disease
Journal of Neuroimmune Pharmacology (2025)
-
Neuroprotective Effects of Piceatannol on Olfactory Bulb Injury after Subarachnoid Hemorrhage
Molecular Neurobiology (2023)
-
High plasma levels of pro-inflammatory factors interleukin-17 and interleukin-23 are associated with poor outcome of cardiac-arrest patients: a single center experience
BMC Cardiovascular Disorders (2020)
-
Ganciclovir reduces irinotecan-induced intestinal toxicity by inhibiting NLRP3 activation
Cancer Chemotherapy and Pharmacology (2020)
-
TLR4 deficiency has a protective effect in the MPTP/probenecid mouse model of Parkinson’s disease
Acta Pharmacologica Sinica (2019)